Achalasia
|
WikiDoc Resources for Achalasia |
|
Articles |
|---|
|
Most recent articles on Achalasia |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Achalasia at Clinical Trials.gov Clinical Trials on Achalasia at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Achalasia
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Achalasia Discussion groups on Achalasia Directions to Hospitals Treating Achalasia Risk calculators and risk factors for Achalasia
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Achalasia |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
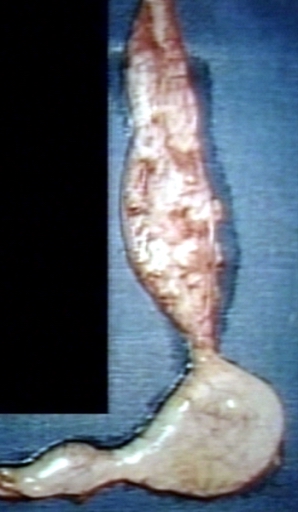
Achalasia, also known as esophageal achalasia, achalasia cardiae, cardiospasm, dyssynergia esophagus, and esophageal aperistalsis, is an esophageal motility disorder.[1] In this disorder, the smooth muscle layer of the esophagus has impaired peristalsis (muscular ability to move food down the esophagus), and the lower esophageal sphincter (LES) fails to relax properly in response to swallowing.[2] The most common form is primary achalasia, which has no known underlying cause. However, a small proportion occurs as a secondary result of other conditions, such as esophageal cancer or (in South America) Chagas disease. As a summary:
- The term achalasia is Greek for ‘does not relax’.
- During normal swallowing, a peristaltic wave propels food down the esophagus and initiates relaxation of the lower esophageal sphincter (LES).
- In achalasia, there is a loss of distal esophageal peristalsis and the LES fails to relax.
- Thankfully, achalasia is a rare disease, with an incidence of ~ 1 case per 100,000. Men and women are equally affected. Onset can be at any age, however it is rare to be seen before adolescence, and is most commonly seen in people between 25 and 60 years old.
Pathophysiology & Etiology
- Achalasia results from degeneration of neurons in the esophageal wall.
- There is a decrease in the number of ganglion cells in the myenteric plexus, and the ganglion cells that remain, have been found to be associated with a lymphocytic inflammatory response.
- The neurons that are destroyed are predominantly inhibitory neurons, which act via release of nitric oxide, with a relative sparing of the cholinergic neurons responsible for smooth muscle contraction.
- It has also been shown that some patients have destruction of the dorsal motor nucleus of the vagus in the brainstem, as well as Wallerian degeneration of the vagal fibers that supply the esophagus.
- The final result is an increase in LES tone and loss of normal peristalsis.
- Patients with achalasia can also have incomplete relaxation of the UES and stomach.
- The etiology of achalasia is unknown.
- Theories include:
- Autoimmune
- Two-thirds of patients have auto-antibodies directed against DARP-32, a dopamine-carrying protein on the surface of cells in the myenteric plexus. Achalasia is also associated with HLA-DQw1.
- Infection
- Several diseases have been associated with motor abnormalities similar or identical to those of achalasia and have been called pseudoachalasia.
- Malignancy (especially gastric, lung, lymphoma and pancreatic) causes achalasia by either direct invasion of the esophageal neuronal plexus, or via the release of unidentified evil humors that act in a paraneoplastic function.
- Chagas disease: Trypanosoma cruzi directly infects the esophagus.
- Amyloidosis, sarcoidosis, eosinophilic gastroenteritis, neurofibromatosis, juvenile Sjögren’s, Ogilvie’s syndrome and Anderson-Fabry’s disease have also been associated with pseudoachalasia.
- Autoimmune
History and Symptoms
- Primarily result from the increased tone of the LES.
- Regurgitation of undigested food
- Coughing, especially when reclining or lying down
- Weight loss due to inadequate nutrient intake
- Non-cardiac chest pains (NCCP), which may radiate to the back, jaw, neck, and arms
- Some patients also experience heartburn or frequent hiccups
- Dysphagia for solids and liquids is the most common feature, being seen in 91 % and 85% respectively.
- The specificity of dysphagia for liquids is relatively high, although it can be seen in other diseases such as progressive systemic sclerosis.
- Due to the slowly progressive nature of the disease, many patients have symptoms for years prior to seeking medical attention (mean ~ 4.7 years in one study).
- Difficulty belching is seen in 85 % of patients and likely results from failure of the UES to relax.
- 40 % of patients describe weight loss, regurgitation, chest pain and heartburn.
- Patients often adopt certain behaviors to enhance esophageal emptying such as lifting the neck or throwing their shoulders back.
- The incidence of esophageal cancer is controversial in patients with achalasia. Some Swedish studies report an increased incidence, and suggest routine surveillance esophago-gastroduodenoscopy (EGD). This has not been shown to be the case in the U.S., and current recommendations do not include routine EGD.
Differential Diagnosis
In alphabetical order. [3] [4]
- Amyloidosis
- Bronchial carcinoma
- Chagas disease
- Chronic idiopathic intestinal pseudo-obstruction
- Drugs
- Gastric carcinoma
- Herpes Zoster
- Hodgkin's Lymphoma
- Idiopathic achalasia
- Ischemia
- Mesothelioma
- Non-Hodgkin's lymphoma
- Postvagotomy
- Prostate cancer
- Radiation therapy
- Sarcoidosis
- Toxins
Diagnosis
Due to the similarity of symptoms, achalasia can be misdiagnosed as other disorders, such as gastroesophageal reflux disease (GERD), hiatus hernia, and even psychosomatic disorders.
Investigations for achalasia include
- X-ray with a barium swallow, or esophagography. The patient swallows a barium solution, which fails to pass smoothly through the lower esophageal sphincter. An air-fluid margin is seen over the barium column due to the lack of peristalsis. Narrowing is observed at the level of the gastroesophageal junction ("bird's beak" or "rat tail" appearance of the lower esophagus). Esophageal dilation is present in varying degrees as the esophagus is gradually stretched by retained food. A five-minute timed barium swallow is useful to measure the effectiveness of treatment.
- Fluoroscopy can be used to demonstrate the lack of peristaltic waves in the smooth-muscle portion of the esophagus. It may also reveal ‘vigorous’ achalasia, which is characterized by random spastic contractions in the esophagus.
- Manometry, the key test for establishing the diagnosis. A probe measures the pressure waves in different parts of the esophagus and stomach during the act of swallowing. A thin tube is inserted through the nose, and the patient is instructed to swallow several times.
- Elevated resting LES pressure, usually > 45 mmHg.
- Incomplete relaxation of the LES.
- Aperistalsis – contractions may be absent, diffuse and not coordinated, and / or ‘vigorous’ (> 60 mmHg).
- Most patients should get and EGD – primarily in order to rule out malignancy (esophageal and gastric).
- Findings include a dilated esophagus with residual pill / food fragments, normal mucosa and occasionally candidiasis (due to the prolonged stasis).
- Factors associated with an increased risk of malignancy include symptoms less than 6 months, presentation after 60 years old, excessive weight loss and difficult passage of the endoscope through the gastroesophageal junction.
- As malignancies can be intramural and not visible with the scope, repeat EGDs with biopsy are recommended when there is a ‘high’ suspicion of malignancy.
- Cholecystokinin (CCK) stimulation test: CCK causes mild contraction of the LES and a more pronounced release of inhibitory neurotransmitters in the wall of the esophagus. In normal people, LES tone will decrease due to the predominant effect of the inhibitory neurotransmitters. In patients with achalasia, however, the stimulatory effect on the LES is unopposed, and LES pressure increases.
- Endoscopy, which provides a view inside the esophagus and stomach. A small camera is inserted through the mouth while the patient is under sedation. The endoscopist observes a "pop" as the scope passes through the non-relaxing lower esophageal sphincter.
- CT scan may be used to exclude pseudoachalasia, or achalasia symptoms resulting from a different cause, usually esophageal cancer.
Pathological examination reveals a defect in the nerves that control the motility of the esophagus (the myenteric plexus). The esophagus is dilated and hypertrophied. In Chagas disease, the ganglion cells are destroyed by Trypanosoma cruzi, the causative parasite.[5]
Complications
- Gastroesophageal reflux disease (GERD) or heartburn.
- Achalasia patients have an increased risk of developing Barrett's esophagus or Barrett's mucosa, a premalignant condition which may lead to esophageal cancer over a period of years.
- Aspiration pneumonia: Food and liquid, including saliva, are retained in the esophagus and may be inhaled into the lungs, especially while sleeping in a horizontal position.
Treatment
- Medication:
- Intra-sphincteric injection of botulinum toxin (or botox), to paralyze the lower esophageal sphincter and prevent spasms. As in the case of botox injected for cosmetic reasons, the effect is only temporary, and symptoms return quickly in most patients. Botox injections cause scarring in the sphincter which may increase the difficulty of later Heller myotomy. This therapy is only recommended for elderly patients who cannot risk surgery.
- BoTox acts as a zinc-dependant protease and cleaves a protein called SNAP-25. This results in a block of acetylcholine release from the presynaptic nerve terminal. As it is the excitatory neurons that release acetylcholine, a decrease in LES tone is observed.
- It has also been shown that BoTox interferes with cholinergic signaling in the myenteric nervous system that supplies smooth muscle, and hence also decreases smooth muscle contractility.
- Pasricha et.al. showed that 90% of patients experienced immediate relief, however only 65% have relief at 6 months, and only 42% are symptom free at one year.
- Relief was associated with a reduction in LES pressure by 40%, an increase in esophageal diameter by 17%, and a reduction in esophageal retention of 33%.
- BoTox is very well tolerated, and only ~ 5% develop symptomatic gastroesphageal reflux disease (GERD).
- The technique is currently being refined with even better results when injection is guided by endoscopic ultrasound.
- Some surgeons believe that BoTox can increase the difficulty of future surgery, and therefore recommend it only for patients who are not candidates for dilation or surgery.
- BoTox acts as a zinc-dependant protease and cleaves a protein called SNAP-25. This results in a block of acetylcholine release from the presynaptic nerve terminal. As it is the excitatory neurons that release acetylcholine, a decrease in LES tone is observed.
- Drugs that reduce LES pressure may be useful, especially as a way to buy time while waiting for surgical treatment. These include calcium channel blockers such as nifedipine, and nitrates such as isosorbide dinitrate and nitroglycerin. Unfortunately, many patients experience unpleasant side effects such as headache and swollen feet, and these drugs often stop helping after several months.
- Nitrates,
- Aminophylline,
- Terbutaline
- Ca++ channel blockers
- Decreases LES tone
- Usually only provide minimal relief.
- As the pills themselves can get stuck in the esophagus, this can complicate the disease.
- Balloon (pneumatic) dilation, also called dilatation. The muscle fibers are stretched and slightly torn by forceful inflation of a balloon placed inside the lower esophageal sphincter. Gastroenterologists who specialize in achalasia and have done many of these forceful balloon dilations have better results and fewer perforations than inexperienced ones. There is always a small risk of a perforation which would have to be fixed by surgery right away. Gastroesophageal reflux (GERD) occurs after pneumatic dilation in some patients. Pneumatic dilation causes some scarring which may increase the difficulty of Heller myotomy if this surgery is needed later. Pneumatic dilation is most effective on the long term in patients over the age of 40; the benefits tend to be shorter-lived in younger patients. This treatment may need to be repeated with larger balloons for maximum effectiveness.
- Surgery: Heller myotomy helps 90% of achalasia patients. It can usually be performed by a keyhole approach, or laparoscopically.[6] The myotomy is a lengthwise cut along the esophagus, starting above the LES and extending down onto the stomach a little way. The esophagus is made of several layers, and the myotomy only cuts through the outside muscle layers which are squeezing it shut, leaving the inner muscosal layer intact. A partial fundoplication or "wrap" is added in order to prevent excessive reflux, which can cause serious damage to the esophagus over time. In a Dor (anterior) fundoplication, part of the stomach is laid over the esophagus and stitched in place so whenever the stomach contracts, it also closes off the esophagus instead of squeezing stomach acids into it. After surgery, patients should keep to a soft diet for several weeks to a month, avoiding foods that can aggravate reflux.
- Bougienage
- A barbaric (don’t tell your patients this) mechanical dilation of the LES with a firm rubber hose. This tends to be much more effective for patients with strictures than for achalasia.
- Balloon Dilation
- Also depends on ripping the LES.
- There is a fine line between achieving a good result and causing esophageal perforation (seen in 2-6%).
- Additionally, there are no guidelines concerning inflation time, number of inflations / session, inflation pressure, and how many sessions a patient should have before moving to another therapeutic modality.
- Approximately 60 –85 % of patients have good initial results. Unfortunately, 50 % of patients will require further therapy within the next 5 years.
- There is also data that suggests that repeated attempts are less likely to be successful and are associated with an increased risk of perforation.
- It appears that better results are associated with age > 45 years, patients with symptoms greater than 5 years, and in those with a mildly dilated esophagus.
- The other major side effect is the development of reflux esophagitis in ~ 2%.
- Surgical myotomy was first performed by Heller in 1913. The operation consisted of two myotomies on opposite sides of the esophagus performed through a laparotomy.
- The modified Heller approach (via left thoracotomy) has a success rate of 70–90%, and a mortality rate of 0.3% (similar to the 0.2% for pneumatic dilation).
- Reflux occurs in ~ 10%, and can be complicated by ulceration, stricture and the development of Barrett’s.
- There is little good data comparing surgery vs. pneumatic dilation, however surgery may have a higher rate of long-term benefit (95% vs. 65% at ~5 years).
- Surgery is a good option for younger patients (<40 years old) as balloon dilation is only 50% successful with the 1st treatment, and < 70% effective overall in this age group.
- It is also recommended for patients in whom dilation is especially risky (those with a tortuous distal esophagus, esophageal diverticula or who have had previous surgery of the gastroenterology (GE) junction), and those who have failed BoTox.
- The surgery can also be preformed laparoscopically or thoracoscopically, and early data suggests equivalent short term results when compared with the open procedure.
- Alternative treatments: Some patients have reported temporary improvement with acupuncture, traditional Chinese herbal medicine, or relaxation techniques.
- Lifestyle changes: Achalasia patients need to eat slowly, chew very well, drink plenty of water with meals, and avoid eating near bedtime. It is helpful to sleep with the head elevated by raising the head of the bed or using a wedge pillow. Proton pump inhibitors may help prevent reflux damage after surgery by inhibiting gastric acid secretion. Foods that can aggravate reflux, including ketchup and other tomato products, citrus fruits, chocolate, mint, alcohol, and caffeine, should also be avoided.
- Follow-up monitoring: Even after successful treatment of achalasia, swallowing may still deteriorate over time. It's important to check every year or two with a timed barium swallow because some may need pneumatic dilations, a repeat myotomy, or even esophagectomy after many years. Some doctors recommend pH testing and endoscopy to check for reflux damage, which may lead to a stricture or cancer of the esophagus if untreated.
See also
References
"Achalasia." Society of Surgeons of the Alimentary Tract. http://www.guideline.gov/ 1996-2000. Retrieved information about Barrett's syndrome and link of predisposition to adenocarcinoma and squamous cell cancer April 4, 2007.
- ↑ Kraichely R, Farrugia G (2006). "Achalasia: physiology and etiopathogenesis". Dis Esophagus. 19 (4): 213–23. PMID 16866850.
- ↑ Park W, Vaezi M (2005). "Etiology and pathogenesis of achalasia: the current understanding". Am J Gastroenterol. 100 (6): 1404–14. PMID 15929777.
- ↑ Sailer, Christian, Wasner, Susanne. Differential Diagnosis Pocket. Hermosa Beach, CA: Borm Bruckmeir Publishing LLC, 2002:77 ISBN 1591032016
- ↑ Kahan, Scott, Smith, Ellen G. In A Page: Signs and Symptoms. Malden, Massachusetts: Blackwell Publishing, 2004:68 ISBN 140510368X
- ↑ Rubin's Pathology - Clinicopathological Foundations of Medicine. Maryland: Lippincott Williams & Wilkins. 2001. pp. p. 665. ISBN 0-7817-4733-3. Unknown parameter
|coauthors=ignored (help) - ↑ Deb S, Deschamps C, Cassivi SD; et al. (2005). "Laparoscopic esophageal myotomy for achalasia: factors affecting functional results". Annals of Thoracic Surgery. 80 (4): 1191–1195. PMID 16181839.
External links
- Achalasia at the Open Directory Project
- U.S. Society for Surgery of the Alimentary Tract Guideline about achalasia.
- achalasia.us a site created to help educate and provide support to those people who suffer from achalasia, and is dedicated in helping them and their families who share in the suffering.
Template:SIB Template:Gastroenterology de:Achalasie it:Acalasia ms:Akalasia nl:Achalasie tl:Achalasia