21-hydroxylase deficiency pathophysiology: Difference between revisions
No edit summary |
|||
| Line 2: | Line 2: | ||
{{Congenital adrenal hyperplasia due to 21-hydroxylase deficiency}} | {{Congenital adrenal hyperplasia due to 21-hydroxylase deficiency}} | ||
{{CMG}} {{ | {{CMG}}; '''Associate Editor-In-Chief:''' {{MJ}} | ||
==Overview== | ==Overview== | ||
Development of [[congenital adrenal hyperplasia]] due to 21-hydroxylase deficiency is the result of a defective [[P450]]c21 enzyme. Genes involved in the pathogenesis of congenital adrenal hyperplasia due to 21-hydroxylase deficiency include the ''CYP21'' gene. | Development of [[congenital adrenal hyperplasia]] due to 21-hydroxylase deficiency is the result of a defective [[P450]]c21 enzyme. Genes involved in the pathogenesis of congenital adrenal hyperplasia due to 21-hydroxylase deficiency include the ''CYP21'' gene. | ||
== Pathophysiology == | == Pathophysiology == | ||
| Line 54: | Line 32: | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Revision as of 15:17, 12 July 2017
|
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency Microchapters |
|
Differentiating Congenital adrenal hyperplasia due to 21-hydroxylase deficiency from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
21-hydroxylase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of 21-hydroxylase deficiency pathophysiology |
|
Directions to Hospitals Treating Congenital adrenal hyperplasia due to 21-hydroxylase deficiency |
|
Risk calculators and risk factors for 21-hydroxylase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Mehrian Jafarizade, M.D [2]
Overview
Development of congenital adrenal hyperplasia due to 21-hydroxylase deficiency is the result of a defective P450c21 enzyme. Genes involved in the pathogenesis of congenital adrenal hyperplasia due to 21-hydroxylase deficiency include the CYP21 gene.
Pathophysiology
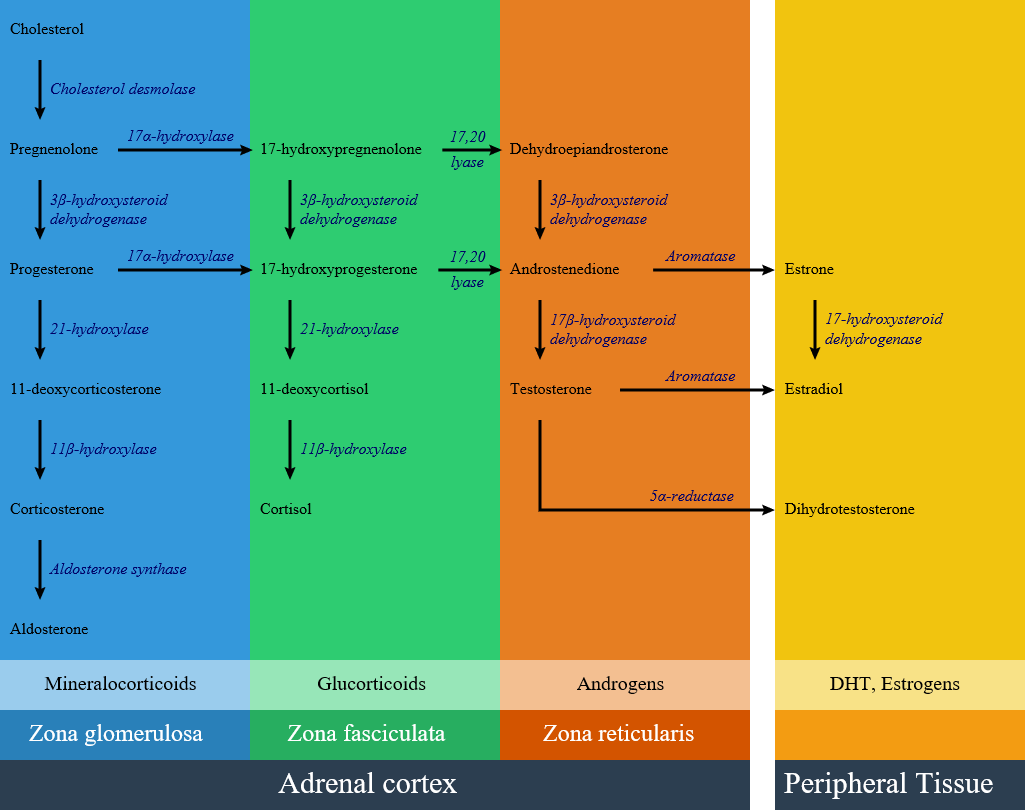
In patients with 21-hydroxylase deficiency, there is a defective conversion of 17-hydroxyprogesterone to 11-deoxycortisol which results in decreased cortisol synthesis and therefore increased corticotropin (ACTH) secretion and as a consequence of rising ACTH there is an increased production of androgens.[1]
More than 95% of all cases of CAH are caused by 21-hydroxylase deficiency (21-OHD);
The clinical manifestation of congenital adrenal hyperplasia is closely related to the type and severity of impairment.
Genetics
- Congenital adrenal hyperplasia subtypes are all autosomal recessive and monogenetic. The disease manifestation follows the allele that results in a more functional enzyme, and generally correlation between genotype and phenotype is good.[2][3][2]
- Responsible gene for 21 OH deficiency is CYP21A. This gene is located within the human leucocyte antigen class III region of chromosome 6. CYP21A gene has two types:
- An active gene called CYP21A2, which encodes 21-hydroxylase, a cytochrome P450 type II enzyme of 495 amino acids.
- The other gene is a non-functional pseudogene named CYP21A1 or CYP21P. This pseudogene produces an enzyme with no activity because it lacks eight bases from codons 110-112, which results in a stop codon[4]
- Meiotic recombination events occurs in this genomic region as a result of the high degree of sequence homology between CYP21A2 and its pseudogene CYP21A1.
- Approximately 70% of CYP21A2 disease is due to gene conversion and micro-deletions in CYP21A1 gen.
- Approximately 25% to 30% are chimeric genes due to large deletions.
- Approximately 1% to 2% of cases are due to de novo mutations because of high variability of the CYP21A2 locus.
- Chromosome 6 uniparental disomy is rare cause of 21-hydroxylase deficiency with an unknown prevalence.
Conventionally, classic 21OH deficiency is subclassified into salt wasting and simple virilising forms, which reflect the severity of aldosterone deficiency. Mutations that completely inactivate CYP21A2 result in the salt-wasting phenotype, which, without neonatal screening, presents in the first 2 weeks of life with a life-threatening adrenal crisis ( table 2).23 Patients with classic simple virilising congenital adrenal hyperplasia have mutations that retain 1–2% of 21OH activity and minimal aldosterone production prevents a neonatal crisis.40 Excess fetal adrenal androgen exposure results in virilisation of external genitalia of 46,XX patients with classic 21OH deficiency (salt wasting and simple virilising; figure 3A). Without neonatal screening, male toddlers with the simple virilising form of the disorder are diagnosed with signs and symptoms of androgen excess. Postnatal excess androgen presence leads to premature growth of pubic hair and rapid skeletal growth in children. Patients with the non-classic form retain up to 50% of enzyme activity and mostly do not have adrenal insufficiency, but might have partial glucocorticoid deficiency, and female patients have normal genitalia.41 Patients might present with mild androgen excess or have few or no symptoms. In fact, the term cryptic congenital adrenal hyperplasia was created to define patients with non-classic congenital adrenal hyperplasia who are identified by family genetic studies, but are otherwise asymptomatic.42
References
- ↑ White PC, Speiser PW (2000). "Congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Endocr. Rev. 21 (3): 245–91. doi:10.1210/edrv.21.3.0398. PMID 10857554.
- ↑ 2.0 2.1 Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP (2011). "Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 96 (1): E161–72. doi:10.1210/jc.2010-0319. PMC 3038490. PMID 20926536.
- ↑ New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T (2013). "Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency". Proc. Natl. Acad. Sci. U.S.A. 110 (7): 2611–6. doi:10.1073/pnas.1300057110. PMC 3574953. PMID 23359698.
- ↑ White PC, New MI, Dupont B (1986). "Structure of human steroid 21-hydroxylase genes". Proc. Natl. Acad. Sci. U.S.A. 83 (14): 5111–5. PMC 323900. PMID 3487786.