Rivaroxaban labels and packages
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Labels and Packages
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
NDC 50458-580-30 30 Tablets
Xarelto® (rivaroxaban) Tablets 10 mg
Rx only
janssen
 |
PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle Label
NDC 50458-578-30 30 Tablets
Xarelto® (rivaroxaban) Tablets 15 mg
Dispense the accompanying Medication Guide to each patient.
janssen
Rx only
 |
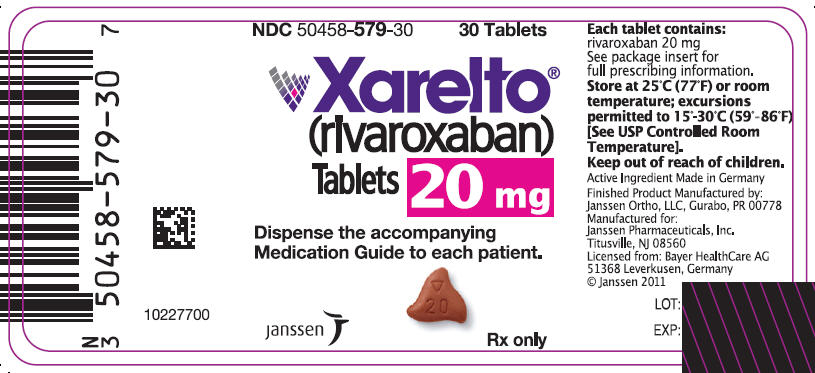
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label NDC 50458-579-30 30 Tablets
Xarelto® (rivaroxaban) Tablets 20 mg
Dispense the accompanying Medication Guide to each patient.
janssen
Rx only
 |
References
Adapted from the FDA Package Insert.