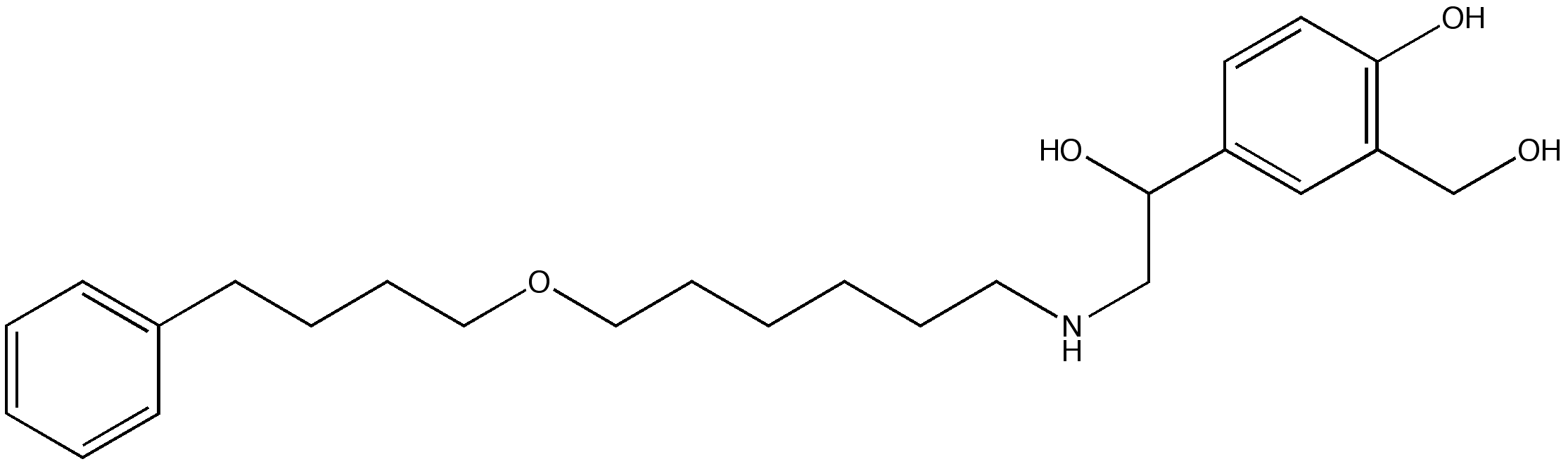
Fluticasone/salmeterol
| Error creating thumbnail: File missing | |
 | |
| Combination of | |
|---|---|
| Fluticasone | Glucocorticoid |
| Salmeterol | Long-Acting Beta2 Agonist |
| [[{{{component3}}}]] | ? Class |
| [[{{{component4}}}]] | ? Class |
| [[{{{component5}}}]] | ? Class |
| Clinical data | |
| Routes of administration | Inhaled |
| Legal status | |
| Legal status |
|
| Identifiers | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
The combination preparation fluticasone/salmeterol is a formulation containing fluticasone propionate and salmeterol xinafoate used in the management of asthma and chronic obstructive pulmonary disease (COPD). It is marketed by GlaxoSmithKline under various trade names including Advair(USA), Seretide(EU), Viani(Germany), Adoair(Japan), and ForAir(India).
Fluticasone, a corticosteroid, is the anti-inflammatory component of the combination, while salmeterol treats constriction of the airways. Together, they relieve the symptoms of coughing, wheezing and shortness of breath better than either fluticasone or salmeterol taken on its own.
Formulations

Advair is available in 3 or 4 dosage strengths, depending on the patient's country, as a DPI (dry powder inhaler). The smallest dosage is 100mcg/50mcg, the intermediate dosage is 250mcg/50mcg and the highest dosage is 500/50. (mcg refers to micrograms)
Advair HFA inhalation aerosol as a MDI (metered dose inhaler) is now available in the US and Canada as Advair HFA 45mcg/21mcg, Advair HFA 115mcg/21mcg, and Advair HFA 230mcg/21mcg. These contain 120 inhalations and are generally prescribed as a 30 day supply. (2 inhalations twice a day)
Internationally the fluticasone/salmeterol combination is delivered by a number of devices, including standard aerosol metered dose inhalers (brand name "Evohaler" in the UK) or dry-powder devices termed "Accuhaler" in the UK and Australia, and "Diskus" in the U.S. These purple disk-shaped containers are about 3.5 inches (8.9 cm) across and about 1 inch thick (2.5 cm). The cleverly designed discus container utilizes a machined 2 piece long foil ribbon with each unit dose held in a small caplet shaped depressions along the entire dose-count-length. Once the lever is actuated the dose is advanced by pealing away the flat outer most layer exposing the medication that is ready to be breathed in.
On August 8, 2007 the FDA issued a "not approved" letter to GlaxoSmithKline (GSK) on the 500/50 strength for the treatment of patients with chronic obstructive pulmonary disease (COPD). The GSK internal notice
Side effects
The common and minor side effects of this combination are those of its individual drugs. For instance, the use of inhaled corticosteroids is associated with oral candidiasis.
Whilst the use of inhaled steroids and long acting beta-adrenoceptor agonist (LABA) are recommended in asthma guidelines for the resulting improved symptom control,[1] concerns have been raised that salmeterol may increase the small risks of asthma deaths and this additional risk is not reduced with the additional use of inhaled steroids.[2] This seems to occur because although LABAs relieve asthma symptoms, they also promote bronchial inflammation and sensitivity without warning.[3]
In a clinical trial, thirteen people died out of a group of 13,176 people taking salmeterol.
Other side effects include increased blood pressure, increase of aggression, change in heart rate, or an irregular heartbeat.
Footnotes
- ↑ British Thoracic Society & Scottish Intercollegiate Guidelines Network (SIGN). British Guideline on the Management of Asthma. Guideline No. 63. Edinburgh:SIGN; 2004. (HTML, Full PDF, Summary PDF)
- ↑ Salpeter S, Buckley N, Ormiston T, Salpeter E (2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Ann Intern Med. 144 (12): 904–12. PMID 16754916.
- ↑ Krishna Ramanujan (2006). "Common asthma inhalers cause up to 80 percent of asthma-related deaths, Cornell and Stanford researchers assert". ChronicalOnline - Cornell University. Unknown parameter
|month=ignored (help)
External links
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs that are a combination of chemicals
- Antiasthmatic drugs
- GlaxoSmithKline brands