Methyldopa injection labels and packages: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
{{Methyldopa}} | {{Methyldopa}} | ||
{{CMG}}; {{AE}} {{AK}} | {{CMG}}; {{AE}} {{AK}} | ||
==Labels and Packages== | |||
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Container | PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Container | ||
Revision as of 20:10, 12 March 2014
| Methyldopa |
|---|
| Methyldopa tablet® FDA Package Insert |
| Indications and Usage |
| Dosage and Administration |
| Contraindications |
| Warnings |
| Precautions |
| Adverse Reactions |
| Drug Interactions |
| Use in Specific Populations |
| Overdosage |
| Description |
| Clinical Pharmacology |
| Nonclinical Toxicology |
| How Supplied/Storage and Handling |
| Labels and Packages |
| Methyldopa injection® FDA Package Insert |
| Indications and Usage |
| Dosage and Administration |
| Contraindications |
| Warnings |
| Precautions |
| Adverse Reactions |
| Drug Interactions |
| Use in Specific Populations |
| Overdosage |
| Description |
| Clinical Pharmacology |
| Nonclinical Toxicology |
| How Supplied/Storage and Handling |
| Labels and Packages |
| Clinical Trials on Methyldopa |
| ClinicalTrials.gov |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Labels and Packages
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Container
NDC 0517-8905-10
METHYLDOPATE HCl INJECTION, USP
250 mg/5 mL (50 mg/mL)
5 mL SINGLE DOSE VIAL
FOR IV USE AFTER DILUTION
Rx Only
AMERICAN REGENT, INC. SHIRLEY, NY 11967
 |
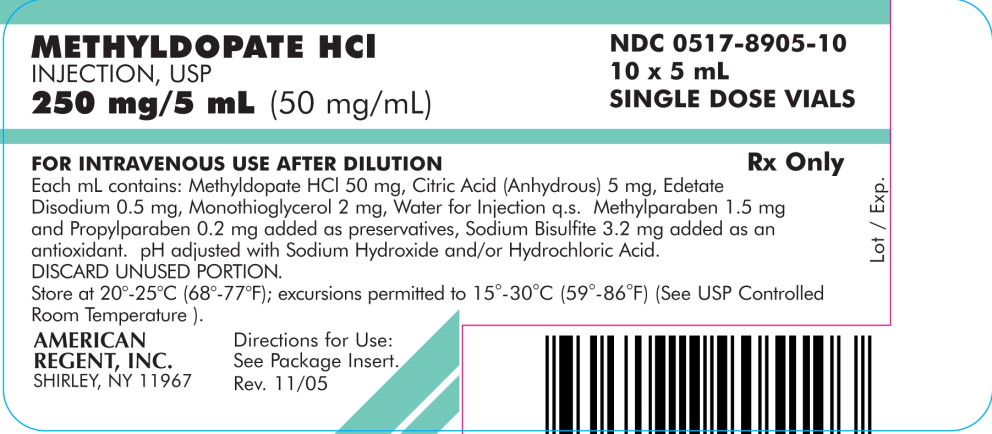
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Carton
PRINCIPAL DISPLAY PANEL – Carton
METHYLDOPATE HCl INJECTION, USP
250 mg/5 mL (50 mg/mL)
NDC 0517-8905-10
10 x 5 mL SINGLE DOSE VIALS
FOR INTRAVENOUS USE AFTER DILUTION
Rx Only
Each mL contains: Methyldopate HCl 50 mg, Citric Acid (Anhydrous) 5 mg, Edetate Disodium 0.5 mg, Monothioglycerol 2 mg, Water for Injection q.s. Methylparaben 1.5 mg and Propylparaben 0.2 mg added as preservatives, Sodium Bisulfite 3.2 mg added as an antioxidant. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid. DISCARD UNUSED PORTION. Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (See USP Controlled Room Temperature). Directions for Use: See Package Insert.
AMERICAN REGENT, INC. SHIRLEY, NY 11967
Rev. 11/05
 |
References
- ↑ "METHYLDOPATE HYDROCHLORIDE INJECTION, SOLUTION [AMERICAN REGENT, INC.]". Retrieved 10 March 2014.