Topiramate drug interactions: Difference between revisions
Jump to navigation
Jump to search
| Line 21: | Line 21: | ||
|} | |} | ||
* Oral contraceptives: Decreased contraceptive efficacy and increased breakthrough bleeding should be considered, especially at doses greater than 200 mg/day . | |||
<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TOPIRAMATE (TOPIRAMATE ) TABLET, FILM COATED [AUROBINDO PHARMA LIMITED] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=32b48ea0-a215-43b8-83b4-a5435a686d68 | publisher = | date = | accessdate = 6 February 2014 }}</ref> | * Metformin is contraindicated with metabolic acidosis, an effect of topiramate. | ||
* Lithium levels should be monitored when coadministered with high-dose topiramate. | |||
* Other carbonic anhydrase inhibitors: Monitor the patient for the appearance or worsening of metabolic acidosis.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TOPIRAMATE (TOPIRAMATE ) TABLET, FILM COATED [AUROBINDO PHARMA LIMITED] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=32b48ea0-a215-43b8-83b4-a5435a686d68 | publisher = | date = | accessdate = 6 February 2014 }}</ref> | |||
==References== | ==References== | ||
Revision as of 23:18, 6 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
For patient information, click here.
Drug Interactions
- As topiramate inhibits carbonic anhydrase, the concomitant use of other inhibitors of carbonic anhydrase (e.g. acetazolamide) may lead to an increased risk of renal stones.
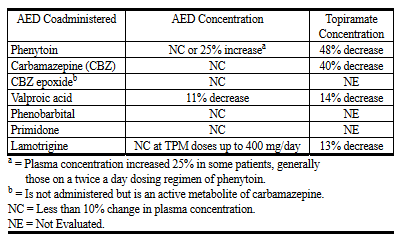
- Enzyme inductors (e.g. carbamazepine) : The elimination of topiramate may be increased, possibly requiring dose escalations of topiramate.
- Phenytoin : Topiramate may increase the plasma-levels of phenytoin.
- Topiramate itself is a weak inhibitor of CYP 2C19 and induces CYP 3A4. Under topiramate a decrease of plasma-levels of estrogens (e.g. 'the pill') and digoxin have been noted.
- Alcohol may cause increased sedation or drowsiness, and increase the risk of having a seizure.
- As listed in the 06/29/2005 label posted at the Drugs@FDA website page 14,'conditions or therapies that predispose to acidosis may be additive to the bicarbonate lowering effects of Topiramate'. Absent from this label is any direct discussion of narcotic (drugs known to promote respiratory acidosis) interactions. This discussion on page 14 is under the topic of Metabolic Acidosis, and is not repeated under the topic of interactions.[1]
 |
- Oral contraceptives: Decreased contraceptive efficacy and increased breakthrough bleeding should be considered, especially at doses greater than 200 mg/day .
- Metformin is contraindicated with metabolic acidosis, an effect of topiramate.
- Lithium levels should be monitored when coadministered with high-dose topiramate.
- Other carbonic anhydrase inhibitors: Monitor the patient for the appearance or worsening of metabolic acidosis.[2]
References
- ↑ [Shttp://www.fda.gov/cder/foi/label/2005/020505s018lbl.pdf FDA.gov]
- ↑ "TOPIRAMATE (TOPIRAMATE ) TABLET, FILM COATED [AUROBINDO PHARMA LIMITED]". Retrieved 6 February 2014.