Delavirdine adverse reactions: Difference between revisions
Jump to navigation
Jump to search
Ahmed Zaghw (talk | contribs) No edit summary |
Ahmed Zaghw (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}}; {{AE}} {{AZ}} | {{CMG}}; {{AE}} {{AZ}} | ||
==ADVERSE REACTIONS== | |||
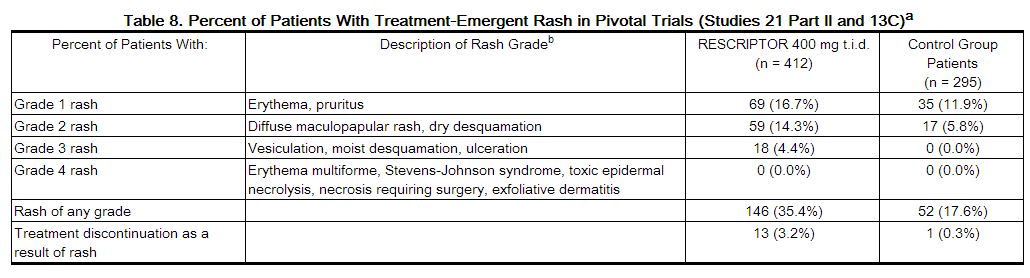
<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = RESCRIPTOR (DELAVIRDINE MESYLATE) TABLET [VIIV HEALTHCARE COMPANY] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb28dcd2-c457-4137-9eec-00062e5a601b | publisher = | date = | accessdate = }}</ref> | The safety of RESCRIPTOR Tablets alone and in combination with other therapies has been studied in approximately 6,000 patients receiving RESCRIPTOR. The majority of adverse events were of mild or moderate (i.e., ACTG Grade 1 or 2) intensity. The most frequently reported drug-related adverse event (i.e., events considered by the investigator to be related to the blinded study medication or events with an unknown or missing causal relationship to the blinded medication) among patients receiving RESCRIPTOR was skin rash.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = RESCRIPTOR (DELAVIRDINE MESYLATE) TABLET [VIIV HEALTHCARE COMPANY] | url =http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb28dcd2-c457-4137-9eec-00062e5a601b | publisher = | date = | accessdate = }}</ref> | ||
{| | |||
|[[File:Deladverse.JPG|800px|thumb]] | |||
|} | |||
==References== | ==References== | ||
Revision as of 23:19, 1 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
ADVERSE REACTIONS
The safety of RESCRIPTOR Tablets alone and in combination with other therapies has been studied in approximately 6,000 patients receiving RESCRIPTOR. The majority of adverse events were of mild or moderate (i.e., ACTG Grade 1 or 2) intensity. The most frequently reported drug-related adverse event (i.e., events considered by the investigator to be related to the blinded study medication or events with an unknown or missing causal relationship to the blinded medication) among patients receiving RESCRIPTOR was skin rash.[1]
 |
References
Adapted from the FDA Package Insert.