Meropenem / vaborbactam (Vabomere): Difference between revisions
No edit summary |
No edit summary |
||
| Line 244: | Line 244: | ||
(Description) | (Description) | ||
|drugBox={{ | |drugBox={{Drugbox | ||
| verifiedrevid = | | Watchedfields = changed | ||
| IUPAC_name = | | verifiedrevid = 462248901 | ||
| image = | | IUPAC_name = (4''R'',5''S'',6''S'')-3-(((3''S'',5''S'')-5-(Dimethylcarbamoyl)pyrrolidin-3-yl)thio)-6-((''R'')-1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | ||
| image = Meropenem.svg | |||
| image2 = Meropenem-from-xtal-1992-3D-balls.png | |||
<!--Clinical data--> | |||
| tradename = Merrem, others | |||
| Drugs.com = {{drugs.com|monograph|meropenem}} | |||
| pregnancy_AU = B2 | |||
| pregnancy_US = B | |||
| legal_AU = S4 | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| routes_of_administration = [[Intravenous]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 100% | |||
| protein_bound = Approximately 2% | |||
| elimination_half-life = 1 hour | |||
| excretion = [[Kidney|Renal]] | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 119478-56-7 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = DH02 | |||
| PubChem = 441130 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00760 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 389924 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = FV9J3JU8B1 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02222 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 43968 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 127 | |||
| PDB_ligand = MEM | |||
<!--Chemical data--> | |||
| C=17 | H=25 | N=3 | O=5 | S=1 | |||
| molecular_weight = 383.464 g/mol | |||
| smiles = O=C3N2\C(=C(\S[C@H]1C[C@@H](C(=O)N(C)C)NC1)[C@H](C)[C@@H]2[C@H]3[C@H](O)C)C(=O)O | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C17H25N3O5S/c1-7-12-11(8(2)21)16(23)20(12)13(17(24)25)14(7)26-9-5-10(18-6-9)15(22)19(3)4/h7-12,18,21H,5-6H2,1-4H3,(H,24,25)/t7-,8-,9+,10+,11-,12-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = DMJNNHOOLUXYBV-PQTSNVLCSA-N | |||
}} | |||
|drugbox={{Drugbox2 | |||
| drug_name = | | drug_name = | ||
| INN = | |||
| type = | |||
| IUPAC_name = {(3''R'',6''S'')-2-Hydroxy-3-[2-(thiophen-2-yl)acetamido]-<br />1,2-oxaborinan-6-yl}acetic acid | |||
| image = Vaborbactam.svg | |||
| width = 250 | |||
| alt = | |||
| caption = | |||
<!--Clinical data--> | <!-- Clinical data --> | ||
| tradename = | | pronounce = | ||
| MedlinePlus = | | tradename = | ||
| licence_US = | | Drugs.com = | ||
| pregnancy_AU = | | MedlinePlus = | ||
| pregnancy_US = | | licence_EU = <!-- EMA requires brand name --> | ||
| | | licence_US = <!-- FDA may use generic name --> | ||
| routes_of_administration = | | DailyMedID = <!-- preference to licence_US --> | ||
| pregnancy_AU = <!-- A/B1/B2/B3/C/D/X --> | |||
| pregnancy_AU_comment = | |||
| pregnancy_US = <!-- A / B / C / D / X / N --> | |||
| pregnancy_US_comment = | |||
| pregnancy_category = | |||
| dependency_liability = | |||
| addiction_liability = | |||
| routes_of_administration = [[Intravenous therapy|IV]] | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_AU_comment = | |||
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_CA_comment = | |||
| legal_DE = <!-- Anlage I, II, III or Unscheduled--> | |||
| legal_DE_comment = | |||
| legal_NZ = <!-- Class A, B, C --> | |||
| legal_NZ_comment = | |||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> | |||
| legal_UK_comment = | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_US_comment = | |||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV--> | |||
| legal_UN_comment = | |||
| legal_status = <!--For countries not listed above--> | |||
<!--Pharmacokinetic data--> | <!-- Pharmacokinetic data --> | ||
| bioavailability = | | bioavailability = | ||
| metabolism = | | protein_bound = | ||
| elimination_half-life = | | metabolism = | ||
| excretion = | | metabolites = | ||
| onset = | |||
| elimination_half-life = | |||
| duration_of_action = | |||
| excretion = | |||
<!--Identifiers--> | <!-- Identifiers --> | ||
| | | CAS_number = 1360457-46-0 | ||
| | | CAS_supplemental = | ||
| | | class = | ||
| | | ATCvet = | ||
| | | ATC_prefix = None | ||
| | | ATC_suffix = | ||
| | | ATC_supplemental = | ||
| | | PubChem = 56649692 | ||
| | | PubChemSubstance = | ||
| | | IUPHAR_ligand = | ||
| | | DrugBank = | ||
| | | ChemSpiderID = 35035409 | ||
| | | UNII = 1C75676F8V | ||
| KEGG = | | KEGG = | ||
| | | ChEBI = | ||
| | | ChEMBL = | ||
| | | NIAID_ChemDB = | ||
| | | synonyms = | ||
<!--Chemical data--> | <!-- Chemical and physical data --> | ||
| C= | H= | N= | O= | | chemical_formula = | ||
| molecular_weight = | | C = 12 | H = 16 | B = 1 | N = 1 | O = 5 | S = 1 | ||
| | | charge = | ||
| | | molecular_weight = | ||
| | | SMILES = B1([C@H](CC[C@H](O1)CC(=O)O)NC(=O)CC2=CC=CS2)O | ||
| | | Jmol = | ||
| | | StdInChI = 1S/C12H16BNO5S/c15-11(7-9-2-1-5-20-9)14-10-4-3-8(6-12(16)17)19-13(10)18/h1-2,5,8,10,18H,3-4,6-7H2,(H,14,15)(H,16,17)/t8-,10-/m0/s1 | ||
| | | StdInChI_comment = | ||
| | | StdInChIKey = IOOWNWLVCOUUEX-WPRPVWTQSA-N | ||
| melting_point = | | density = | ||
| density_notes = | |||
| melting_point = | |||
| melting_high = | |||
| melting_notes = | |||
| boiling_point = | |||
| boiling_notes = | |||
| solubility = | |||
| specific_rotation = | |||
| sec_combustion = | |||
}} | }} | ||
|mechAction= | |mechAction= | ||
*VABOMERE is an antibacterial drug | *VABOMERE is an antibacterial drug | ||
|structure= | |structure= | ||
*Structure of Meropenem Trihydrate | |||
[[image:meropenemstructure.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*Structure of Vaborbactam | |||
[[image:vaborbactamstructure.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|PD= | |PD= | ||
*Similar to other beta-lactam antibacterial drugs, the percentage of time of a dosing interval that unbound plasma concentration of meropenem exceeds the meropenem-vaborbactam minimum inhibitory concentration (MIC) against the infecting organism has been shown to best correlate with efficacy in animal and in vitro models of infection. The ratio of the 24-hour unbound plasma vaborbactam AUC to meropenem-vaborbactam MIC is the index that best predicts efficacy of vaborbactam in combination with meropenem in animal and in vitro models of infection. | *Similar to other beta-lactam antibacterial drugs, the percentage of time of a dosing interval that unbound plasma concentration of meropenem exceeds the meropenem-vaborbactam minimum inhibitory concentration (MIC) against the infecting organism has been shown to best correlate with efficacy in animal and in vitro models of infection. The ratio of the 24-hour unbound plasma vaborbactam AUC to meropenem-vaborbactam MIC is the index that best predicts efficacy of vaborbactam in combination with meropenem in animal and in vitro models of infection. | ||
Revision as of 13:56, 19 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Meropenem / vaborbactam (Vabomere) is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Meropenem / vaborbactam (Vabomere) in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Meropenem / vaborbactam (Vabomere) and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
| File:Meropenem.svg | |
| File:Meropenem-from-xtal-1992-3D-balls.png | |
| Clinical data | |
|---|---|
| Trade names | Merrem, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | Approximately 2% |
| Elimination half-life | 1 hour |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C17H25N3O5S |
| Molar mass | 383.464 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
- VABOMERE is an antibacterial drug
Structure
- Structure of Meropenem Trihydrate
- Structure of Vaborbactam
Pharmacodynamics
- Similar to other beta-lactam antibacterial drugs, the percentage of time of a dosing interval that unbound plasma concentration of meropenem exceeds the meropenem-vaborbactam minimum inhibitory concentration (MIC) against the infecting organism has been shown to best correlate with efficacy in animal and in vitro models of infection. The ratio of the 24-hour unbound plasma vaborbactam AUC to meropenem-vaborbactam MIC is the index that best predicts efficacy of vaborbactam in combination with meropenem in animal and in vitro models of infection.
Pharmacokinetics
Pharmacokinetic (PK) Parameters
- The mean PK parameters of meropenem and vaborbactam in healthy adults with normal renal function after single and multiple 3-hour infusions of VABOMERE 4 grams (meropenem 2 grams and vaborbactam 2 grams) administered every 8 hours are summarized in Table 4.
- The PK parameters of meropenem and vaborbactam were similar for single and multiple dose administration of VABOMERE.
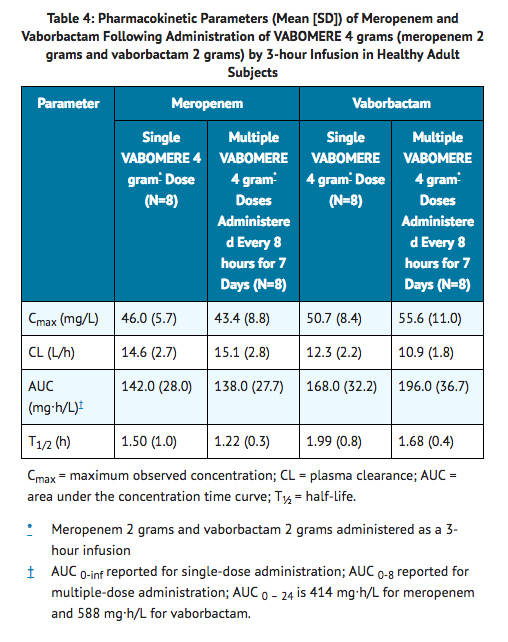
- The maximum plasma concentration (Cmax) and area under the plasma drug concentration time curve (AUC) of meropenem and vaborbactam proportionally increased with dose across the dose range studied (1 gram to 2 grams for meropenem and 0.25 grams to 2 grams for vaborbactam) when administered as a single 3-hour intravenous infusion. There is no accumulation of meropenem or vaborbactam following multiple intravenous infusions administered every 8 hours for 7 days in subjects with normal renal function.
- The mean population PK parameters of meropenem and vaborbactam in 295 patients (including 35 patients with reduced renal function) after 3-hour infusions of VABOMERE 4 grams (meropenem 2 grams and vaborbactam 2 grams) administered every 8 hours (or dose adjusted based on renal function) are summarized in Table 5.
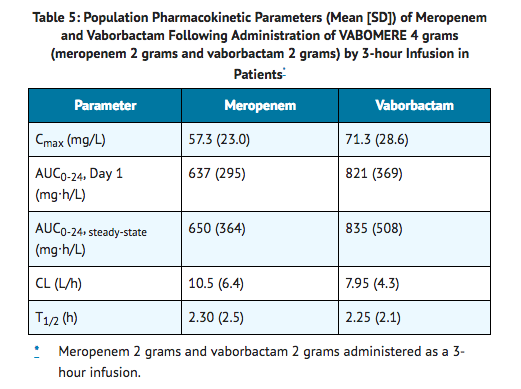
Distribution
- The plasma protein binding of meropenem is approximately 2%. The plasma protein binding of vaborbactam is approximately 33%.
- The steady-state volumes of distribution of meropenem and vaborbactam in patients were 20.2 L and 18.6 L, respectively.
Elimination
- The clearance of meropenem in healthy subjects following multiple doses is 15.1 L/h and for vaborbactam is 10.9 L/h. The t1/2 is 1.22 hours and 1.68 hours for meropenem and vaborbactam, respectively.
Metabolism
- A minor pathway of meropenem elimination is hydrolysis of the beta-lactam ring (meropenem open lactam), which accounts for 22% of a dose eliminated via the urine.
- Vaborbactam does not undergo metabolism.
Excretion
- Both meropenem and vaborbactam are primarily excreted via the kidneys.
- Approximately 40–60% of a meropenem dose is excreted unchanged within 24-48 hours with a further 22% recovered as the microbiologically inactive hydrolysis product. The mean renal clearance for meropenem was 7.8 L/h. The mean non-renal clearance for meropenem was 7.3 L/h which comprises both fecal elimination (~2% of dose) and degradation due to hydrolysis.
- For vaborbactam, 75 to 95% of the dose was excreted unchanged in the urine over a 24 to 48 hour period. The mean renal clearance for vaborbactam was 8.9 L/h. The mean non-renal clearance for vaborbactam was 2.0 L/h indicating nearly complete elimination of vaborbactam by the renal route.
Specific Populations
Patients with Renal Impairment
- Following a single dose of VABOMERE, pharmacokinetic studies with meropenem and vaborbactam in subjects with renal impairment have shown that meropenem AUC0-inf ratios to subjects with normal renal function are 1.28, 2.07, and 4.63 for subjects with mild (eGFR of 60 to 89 mL/min/1.73m2), moderate (eGFR of 30 to 59 mL/min/1.73m2), and severe (eGFR <30 mL/min/1.73m2) renal impairment, respectively; vaborbactam AUC0-inf ratios to subjects with normal renal function are 1.18, 2.31, and 7.8 for subjects with mild, moderate, and severe renal impairment, respectively. Hemodialysis removed 38% of the meropenem dose and 53% of the vaborbactam dose. Vaborbactam exposure was high in subjects with ESRD (eGFR <15 ml/min/1.73 m2). Vaborbactam exposure was higher when VABOMERE was administered after hemodialysis (AUC0-inf ratio to subjects with normal renal function of 37.5) than when VABOMERE was administered before hemodialysis (AUC0-inf ratio to subjects with normal renal function of 10.2).
Patients with Hepatic Impairment
- A pharmacokinetic study conducted with an intravenous formulation of meropenem in patients with hepatic impairment has shown no effects of liver disease on the pharmacokinetics of meropenem.
- Vaborbactam does not undergo hepatic metabolism. Therefore, the systemic clearance of meropenem and vaborbactam is not expected to be affected by hepatic impairment.
Geriatric Patients
- In elderly patients with renal impairment, plasma clearances of meropenem and vaborbactam were reduced, correlating with age-associated reduction in renal function.
Male and Female Patients
- Meropenem and vaborbactam Cmax and AUC were similar between males and females using a population pharmacokinetic analysis.
Racial or Ethnic Groups
- No significant difference in mean meropenem or vaborbactam clearance was observed across race groups using a population pharmacokinetic analysis.
Drug Interactions
- No drug-drug interaction was observed between meropenem and vaborbactam in clinical studies with healthy subjects.
- Based upon the in vitro and in vivo data available to date, there is a low potential for clinically significant drug interactions with vaborbactam.
- Vaborbactam at clinically relevant concentrations does not inhibit the cytochrome P450 isoforms CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 in vitro human liver microsomes. Vaborbactam showed no potential for in vitro induction of CYP1A2, CYP2B6, and CYP3A4 in human hepatocytes. Studies evaluating the potential for meropenem to interact with CYP450 enzymes or active transport systems have not been conducted. However, carbapenems as a class have not shown the potential for inhibition or induction CYP450 enzymes and clinical experience suggests that such effects are unlikely.
- Vaborbactam does not inhibit the following hepatic and renal transporters in vitro at clinically relevant concentrations: P-gp, BCRP, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3 or BSEP. Vaborbactam was not a substrate of OAT1, OAT3, OCT2, P-gp, and BCRP.
- Meropenem is a substrate of OAT1 and OAT3 and as such, probenecid competes with meropenem for active tubular secretion and thus inhibits the renal excretion of meropenem. Following administration of probenecid with meropenem, the mean systemic exposure increased 56% and the mean elimination half-life increased 38%.
- Concomitant administration of meropenem and valproic acid has been associated with reductions in valproic acid concentrations with subsequent loss in seizure control.
Microbiology
Mechanism of Action
- The meropenem component of VABOMERE is a penem antibacterial drug. The bactericidal action of meropenem results from the inhibition of cell wall synthesis. Meropenem penetrates the cell wall of most gram-positive and gram-negative bacteria to bind penicillin-binding protein (PBP) targets. Meropenem is stable to hydrolysis by most beta-lactamases, including penicillinases and cephalosporinases produced by gram-negative and gram-positive bacteria, with the exception of carbapenem hydrolyzing beta-lactamases.
- The vaborbactam component of VABOMERE is a non-suicidal beta-lactamase inhibitor that protects meropenem from degradation by certain serine beta-lactamases such as Klebsiella pneumoniae carbapenemase (KPC). Vaborbactam does not have any antibacterial activity. Vaborbactam does not decrease the activity of meropenem against meropenem-susceptible organisms.
Resistance
- Mechanisms of beta-lactam resistance may include the production of beta-lactamases, modification of PBPs by gene acquisition or target alteration, up-regulation of efflux pumps, and loss of outer membrane porin. VABOMERE may not have activity against gram-negative bacteria that have porin mutations combined with overexpression of efflux pumps.
- Clinical isolates may produce multiple beta-lactamases, express varying levels of beta-lactamases, or have amino acid sequence variations, and other resistance mechanisms that have not been identified.
- Culture and susceptibility information and local epidemiology should be considered in selecting or modifying antibacterial therapy.
- VABOMERE demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and extended-spectrum beta-lactamases (ESBLs) of the following groups: KPC, SME, TEM, SHV, CTX-M, CMY, and ACT. VABOMERE is not active against bacteria that produce metallo-beta lactamases or oxacillinases with carbapenemase activity.
- In the Phase 3 cUTI trial with VABOMERE, some isolates of E. coli, K. pneumoniae, E. cloacae, C. freundii, P. mirabilis, P. stuartii that produced beta-lactamases, were susceptible to VABOMERE (minimum inhibitory concentration ≤4 mcg /mL). These isolates produced one or more beta-lactamases of the following enzyme groups: OXA (non-carbapenemases), KPC, CTX-M, TEM, SHV, CMY, and ACT.
- Some beta-lactamases were also produced by an isolate of K. pneumoniae that was not susceptible to VABOMERE (minimum inhibitory concentration ≥32 mcg/mL). This isolate produced beta-lactamases of the following enzyme groups: CTX-M, TEM, SHV, and OXA.
- No cross-resistance with other classes of antimicrobials has been identified. Some isolates resistant to carbapenems (including meropenem) and to cephalosporins may be susceptible to VABOMERE.
Interaction with Other Antimicrobials
- In vitro synergy studies have not demonstrated antagonism between VABOMERE and levofloxacin, tigecycline, polymyxin, amikacin, vancomycin, azithromycin, daptomycin, or linezolid.
Activity against Meropenem Non-susceptible Bacteria in Animal Infection Models
- Activity against Meropenem Non-susceptible Bacteria in Animal Infection Models
Antimicrobial Activity
- VABOMERE has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections.
Antimicrobial Activity
- VABOMERE has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see INDICATIONS AND USAGE (1.1)].
- Gram-negative bacteria:
- Enterobacter cloacae species complex
- Escherichia coli
- Klebsiella pneumoniae
- The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro MIC less than or equal to the susceptible breakpoint for VABOMERE against isolates of a similar genus or organism group. However, the efficacy of VABOMERE in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials.
- Gram-negative bacteria:
- Citrobacter freundii
- Citrobacter koseri
- Enterobacter aerogenes
- Klebsiella oxytoca
- Morganella morganii
- Proteus mirabilis
- Providencia spp.
- Pseudomonas aeruginosa
- Serratia marcescens
Susceptibility Test Methods
- For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Long-term carcinogenicity studies have not been performed with VABOMERE, meropenem, or vaborbactam.
Mutagenesis
Meropenem
- Genetic toxicity studies were performed with meropenem using the bacterial reverse mutation test, the Chinese hamster ovary HGPRT assay, cultured human lymphocytes cytogenic assay, and the mouse micronucleus test. There was no evidence of mutation potential found in any of these tests.
Vaborbactam
- Genetic toxicity studies were performed with vaborbactam using the bacterial reverse mutation test, chromosomal aberration test and the mouse micronucleus test. There was no evidence of mutagenic potential found in any of these tests.
Impairment of Fertility
Meropenem
- Reproductive studies were performed with meropenem in male and female rats at doses up to 1000 mg/kg/day with no evidence of impaired fertility (approximately equivalent to 1.6 times the MRHD based on body surface area comparison).
- In a reproductive study in cynomolgus monkeys at doses of meropenem up to 360 mg/kg/day (on the basis of body surface area comparison, approximately equivalent to 1.2 times the MRHD) no reproductive toxicity was seen.
Vaborbactam
- Vaborbactam had no adverse effect on fertility in male and female rats at doses up to 1000 mg/kg/day, which is equivalent to approximately 1.6 times the MRHD based on body surface area comparison.
Clinical Studies
Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
- A total of 545 adults with cUTI, including pyelonephritis were randomized into a double-blind, double dummy, multi-center trial comparing VABOMERE (meropenem 2 grams and vaborbactam 2 grams) to piperacillin/tazobactam (piperacillin 4 grams/tazobactam 0.5 grams) intravenously every 8 hours. Switch to an oral antibacterial drug, such as levofloxacin was allowed after a minimum of 15 doses of IV therapy.
- The microbiologically modified intent to treat population (m-MITT) included all randomized patients who received any study drug and had at least 1 baseline uropathogen. Clinical and microbiological response at the end of IV treatment (EOIVT) required both a clinical outcome of cure or improvement and a microbiologic outcome of eradication (all baseline uropathogens at >105 CFU/mL are to be reduced to <104 CFU/mL). Clinical and microbiological response was also assessed at the Test of Cure (TOC) visit (approximately 7 days after completion of treatment) in the m-MITT population and required both a clinical outcome of cure and a microbiological outcome of eradication.
- Patient demographic and baseline characteristics were balanced between treatment groups in the m-MITT population. Approximately 93% of patients were Caucasian and 66% were females in both treatment groups. The mean age was 54 years with 32% and 42% patients greater than 65 years of age in VABOMERE and piperacillin/tazobactam treatment groups, respectively. Mean body mass index was approximately 26.5 kg/m2 in both treatment groups. Concomitant bacteremia was identified in 12 (6%) and 15 (8%) patients at baseline in VABOMERE and piperacillin/tazobactam treatment groups respectively. The proportion of patients with diabetes mellitus at baseline was 17% and 19% in VABOMERE and piperacillin/tazobactam treatment groups, respectively. The majority of patients (approximately 90%) were enrolled from Europe, and approximately 2% of patients were enrolled from North America. Overall, in both treatment groups, 59% of patients had pyelonephritis and 40% had cUTI, with 21% and 19% of patients having a non-removable and removable source of infection, respectively.
- Mean duration of IV treatment in both treatment groups was 8 days and mean total treatment duration (IV and oral) was 10 days; patients with baseline bacteremia could receive up to 14 days of therapy. Approximately 10% of patients in each treatment group in the m-MITT population had a levofloxacin-resistant pathogen at baseline and received levofloxacin as the oral switch therapy. This protocol violation may have impacted the assessment of the outcomes at the TOC visit. These patients were not excluded from the analysis presented in Table 6, as the decision to switch to oral levofloxacin was based on post-randomization factors.
- VABOMERE demonstrated efficacy with regard to clinical and microbiological response at the EOIVT visit and TOC visits in the m-MITT population as shown in Table 6.
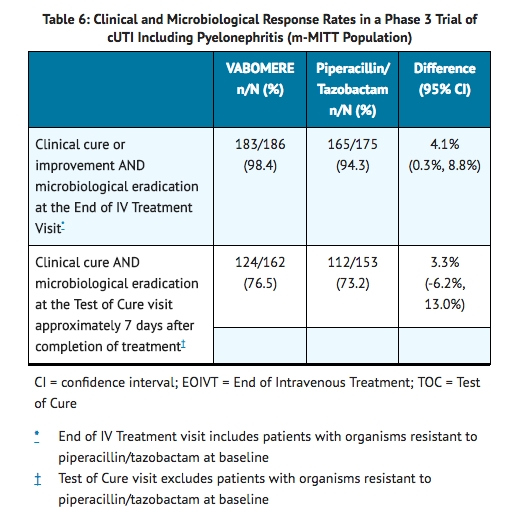
- In the m-MITT population, the rate of clinical and microbiological response in VABOMERE- treated patients with concurrent bacteremia at baseline was 10/12 (83.3%).
- In a subset of the E. coli and K. pneumoniae isolates, genotypic testing identified certain ESBL groups (e.g., TEM, CTX-M, SHV and OXA) in both treatment groups of the Phase 3 cUTI trial. The rates of clinical and microbiological response were similar in the ESBL-positive and ESBL-negative subset at EOIVT; at TOC, clinical and microbiological response was lower in the ESBL-positive as compared to ESBL-negative subset in both treatment groups.
How Supplied
- VABOMERE 2 grams (meropenem and vaborbactam) for injection is supplied as a white to light yellow sterile powder for constitution in single-dose, clear glass vials (NDC 70842-120-01) sealed with a rubber stopper (not made with natural rubber latex) and an aluminum overseal. Each vial is supplied in cartons of 6 vials (NDC 70842-120-06).
- Each vial contains 1 gram of meropenem (equivalent to 1.14 grams of meropenem trihydrate), 1 gram of vaborbactam, and 0.575 gram of sodium carbonate.
Storage
- Store VABOMERE vials at 20°C to 25°C (68°F to 77°F); excursions are permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Meropenem / vaborbactam (Vabomere) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Meropenem / vaborbactam (Vabomere) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Serious Allergic Reactions
- Advise patients that allergic reactions, including serious allergic reactions, could occur and that serious reactions require immediate treatment. Ask patient about any previous hypersensitivity reactions to VABOMERE (meropenem and vaborbactam), penicillins, cephalosporins, other beta-lactams, or other allergens.
Seizures
- Patients receiving VABOMERE on an outpatient basis must be alerted of adverse events such as seizures, delirium, headaches and/or paresthesias that could interfere with mental alertness and/or cause motor impairment. Until it is reasonably well established that VABOMERE is well tolerated, patients should not operate machinery or motorized vehicles.
Potentially Serious Diarrhea
- Counsel patients that diarrhea is a common problem caused by antibacterial drugs including VABOMERE, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
Interaction with Valproic Acid
- Counsel patients to inform their physician if they are taking valproic acid or divalproex sodium. Valproic acid concentrations in the blood may drop below the therapeutic range upon co-administration with VABOMERE. If treatment with VABOMERE is necessary and continued, alternative or supplemental anti-convulsant medication to prevent and/or treat seizures may be needed.
Antibacterial Resistance
- Counsel patients that antibacterial drugs, including VABOMERE, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When VABOMERE is prescribed to treat a bacterial infection, tell patients that although it is common to feel better early in the course of therapy, take the medication exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by VABOMERE or other antibacterial drugs in the future.
Precautions with Alcohol
Alcohol-Meropenem / vaborbactam (Vabomere) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Vabomere
Look-Alike Drug Names
There is limited information regarding Meropenem / vaborbactam (Vabomere) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.