Latanoprostene bunod: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= {{Sonya}} |genericName=generic name |aOrAn=a |drugClass=Acetylcholine release inhibitor, Adrenergic receptor agonist |indicationType=(t...") |
No edit summary |
||
| Line 197: | Line 197: | ||
(Description) | (Description) | ||
|useInPregnancyFDA=( | |useInPregnancyFDA= | ||
=====Risk Summary===== | |||
*There are no available human data for the use of VYZULTA during pregnancy to inform any drug associated risks. | |||
*Latanoprostene bunod has caused miscarriages, abortion, and fetal harm in rabbits. Latanoprostene bunod was shown to be abortifacient and teratogenic when administered intravenously (IV) to pregnant rabbits at exposures ≥ 0.28 times the clinical dose. Doses ≥ 20 μg/kg/day (23 times the clinical dose) produced 100% embryofetal lethality. Structural abnormalities observed in rabbit fetuses included anomalies of the great vessels and aortic arch vessels, domed head, sternebral and vertebral skeletal anomalies, limb hyperextension and malrotation, abdominal distension and edema. Latanoprostene bunod was not teratogenic in the rat when administered IV at 150 mcg/kg/day (87 times the clinical dose). | |||
*The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies. | |||
=====Data (Animal)===== | |||
*Embryofetal studies were conducted in pregnant rabbits administered latanoprostene bunod daily by intravenous injection on gestation days 7 through 19, to target the period of organogenesis. The doses administered ranged from 0.24 to 80 mcg/kg/day. Abortion occurred at doses ≥ 0.24 mcg/kg/day latanoprostene bunod (0.28 times the clinical dose, on a body surface area basis, assuming 100% absorption). Embryofetal lethality (resorption) was increased in latanoprostene bunod treatment groups, as evidenced by increases in early resorptions at doses ≥ 0.24 mcg/kg/day and late resorptions at doses ≥ 6 mcg/kg/day (approximately 7 times the clinical dose). No fetuses survived in any rabbit pregnancy at doses of 20 mcg/kg/day (23 times the clinical dose) or greater. Latanoprostene bunod produced structural abnormalities at doses ≥ 0.24 mcg/kg/day (0.28 times the clinical dose). Malformations included anomalies of sternum, coarctation of the aorta with pulmonary trunk dilation, retroesophageal subclavian artery with absent brachiocephalic artery, domed head, forepaw hyperextension and hindlimb malrotation, abdominal distention/edema, and missing/fused caudal vertebrae. | |||
*An embryofetal study was conducted in pregnant rats administered latanoprostene bunod daily by intravenous injection on gestation days 7 through 17, to target the period of organogenesis. The doses administered ranged from 150 to 1500 mcg/kg/day. Maternal toxicity was produced at 1500 mcg/kg/day (870 times the clinical dose, on a body surface area basis, assuming 100% absorption), as evidenced by reduced maternal weight gain. Embryofetal lethality (resorption and fetal death) and structural anomalies were produced at doses ≥ 300 mcg/kg/day (174 times the clinical dose). Malformations included anomalies of the sternum, domed head, forepaw hyperextension and hindlimb malrotation, vertebral anomalies and delayed ossification of distal limb bones. A no observed adverse effect level (NOAEL) was established at 150 mcg/kg/day (87 times the clinical dose) in this study. | |||
|useInLaborDelivery=(Description) | |useInLaborDelivery=(Description) | ||
|useInNursing= | |useInNursing= | ||
|useInPed= | =====Risk Summary===== | ||
|useInGeri= | *There are no data on the presence of VYZULTA in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for VYZULTA, and any potential adverse effects on the breastfed infant from VYZULTA. | ||
|useInGender= | |useInPed= | ||
|useInRace= | *Use in pediatric patients aged 16 years and younger is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use. | ||
|useInRenalImpair= | |useInGeri= | ||
|useInHepaticImpair= | *No overall clinical differences in safety or effectiveness have been observed between elderly and other adult patients. | ||
|useInReproPotential= | |useInGender= | ||
|useInImmunocomp= | |useInRace= | ||
| | |useInRenalImpair= | ||
|useInHepaticImpair= | |||
|useInReproPotential= | |||
|monitoring= | |useInImmunocomp= | ||
|administration= | |||
*If VYZULTA is to be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure, administer each drug product at least five (5) minutes apart. | |||
*One drop in the affected eye(s) once daily in the evening | |||
|monitoring= | |||
*A reduction in intraocular pressure is evidence of efficacy. | |||
|overdose= | |||
|overdose= | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| | | drug_name = Latanoprostene bunod | ||
| IUPAC_name = | | INN = | ||
| image = | | type =<!-- empty --> | ||
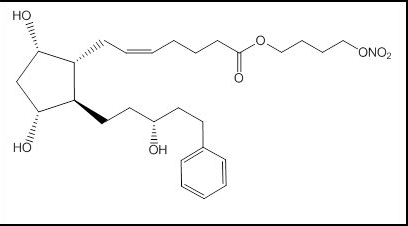
| | | IUPAC_name = 4-Nitrooxybutyl (''Z'')-7-[(1''R'',2''R'',3''R'',5''S'')-3,5-dihydroxy-2-[(3''R'')-3-hydroxy-5-phenylpentyl]cyclopentyl]hept-5-enoate | ||
| image = Latanoprostene bunod.svg | |||
<!--Clinical data--> | | alt = | ||
| tradename = | | caption = | ||
| MedlinePlus = | <!-- Clinical data --> | ||
| | | pronounce = | ||
| | | tradename = Vyzulta | ||
| pregnancy_US = | | Drugs.com = | ||
| | | MedlinePlus = | ||
| routes_of_administration = | | pregnancy_AU = <!-- A/B1/B2/B3/C/D/X --> | ||
| pregnancy_AU_comment = | |||
<!--Pharmacokinetic data--> | | pregnancy_US = <!-- A/B/C/D/X/N --> | ||
| bioavailability = | | pregnancy_category= | ||
| metabolism = | | routes_of_administration = | ||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_AU_comment = | |||
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F--> | |||
| legal_BR_comment = | |||
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_DE = <!-- Anlage I, II, III --> | |||
| legal_NZ = <!-- Class A, B, C --> | |||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> | |||
| legal_US = Rx-only | |||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV--> | |||
| legal_status = <!-- Free text --> | |||
<!-- Pharmacokinetic data --> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| metabolites = | |||
| onset = | |||
| elimination_half-life = | | elimination_half-life = | ||
| excretion = | | duration_of_action = | ||
| excretion = | |||
<!-- Identifiers --> | |||
| CAS_number = 860005-21-6 | |||
| class = | |||
| ATCvet = | |||
| ATC_prefix = <!-- 'none' if uncategorised --> | |||
| ATC_suffix = | |||
| PubChem = | |||
| DrugBank = | |||
| UNII = I6393O0922 | |||
| KEGG = D10441 | |||
| synonyms = BOL-303259-X | |||
<!-- Chemical and physical data --> | |||
| C=27|H=41|N=1|O=8 | |||
| StdInChIKey = LOVMMUBRQUFEAH-UIEAZXIASA-N | |||
| StdInChI=1S/C27H41NO8/c29-22(15-14-21-10-4-3-5-11-21)16-17-24-23(25(30)20-26(24)31)12-6-1-2-7-13-27(32)35-18-8-9-19-36-28(33)34/h1,3-6,10-11,22-26,29-31H,2,7-9,12-20H2/b6-1-/t22-,23+,24+,25-,26+/m0/s1 | |||
| smiles = C1[C@H]([C@@H]([C@H]([C@H]1O)C/C=C\CCCC(=O)OCCCCO[N+](=O)[O-])CC[C@H](CCC2=CC=CC=C2)O)O | |||
}} | |||
|mechAction= | |mechAction= | ||
*Latanoprostene bunod is thought to lower intraocular pressure by increasing outflow of aqueous humor through both the trabecular meshwork and uveoscleral routes. Intraocular pressure is a major modifiable risk factor for glaucoma progression. Reduction of intraocular pressure reduces risk of glaucomatous visual field loss. | *Latanoprostene bunod is thought to lower intraocular pressure by increasing outflow of aqueous humor through both the trabecular meshwork and uveoscleral routes. Intraocular pressure is a major modifiable risk factor for glaucoma progression. Reduction of intraocular pressure reduces risk of glaucomatous visual field loss. | ||
Revision as of 17:56, 17 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
{{{blackBoxWarningTitle}}}
See full prescribing information for complete Boxed Warning.
{{{blackBoxWarningBody}}}
|
Overview
Latanoprostene bunod is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
{{{blackBoxWarningTitle}}}
See full prescribing information for complete Boxed Warning.
{{{blackBoxWarningBody}}}
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Risk Summary
- There are no available human data for the use of VYZULTA during pregnancy to inform any drug associated risks.
- Latanoprostene bunod has caused miscarriages, abortion, and fetal harm in rabbits. Latanoprostene bunod was shown to be abortifacient and teratogenic when administered intravenously (IV) to pregnant rabbits at exposures ≥ 0.28 times the clinical dose. Doses ≥ 20 μg/kg/day (23 times the clinical dose) produced 100% embryofetal lethality. Structural abnormalities observed in rabbit fetuses included anomalies of the great vessels and aortic arch vessels, domed head, sternebral and vertebral skeletal anomalies, limb hyperextension and malrotation, abdominal distension and edema. Latanoprostene bunod was not teratogenic in the rat when administered IV at 150 mcg/kg/day (87 times the clinical dose).
- The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data (Animal)
- Embryofetal studies were conducted in pregnant rabbits administered latanoprostene bunod daily by intravenous injection on gestation days 7 through 19, to target the period of organogenesis. The doses administered ranged from 0.24 to 80 mcg/kg/day. Abortion occurred at doses ≥ 0.24 mcg/kg/day latanoprostene bunod (0.28 times the clinical dose, on a body surface area basis, assuming 100% absorption). Embryofetal lethality (resorption) was increased in latanoprostene bunod treatment groups, as evidenced by increases in early resorptions at doses ≥ 0.24 mcg/kg/day and late resorptions at doses ≥ 6 mcg/kg/day (approximately 7 times the clinical dose). No fetuses survived in any rabbit pregnancy at doses of 20 mcg/kg/day (23 times the clinical dose) or greater. Latanoprostene bunod produced structural abnormalities at doses ≥ 0.24 mcg/kg/day (0.28 times the clinical dose). Malformations included anomalies of sternum, coarctation of the aorta with pulmonary trunk dilation, retroesophageal subclavian artery with absent brachiocephalic artery, domed head, forepaw hyperextension and hindlimb malrotation, abdominal distention/edema, and missing/fused caudal vertebrae.
- An embryofetal study was conducted in pregnant rats administered latanoprostene bunod daily by intravenous injection on gestation days 7 through 17, to target the period of organogenesis. The doses administered ranged from 150 to 1500 mcg/kg/day. Maternal toxicity was produced at 1500 mcg/kg/day (870 times the clinical dose, on a body surface area basis, assuming 100% absorption), as evidenced by reduced maternal weight gain. Embryofetal lethality (resorption and fetal death) and structural anomalies were produced at doses ≥ 300 mcg/kg/day (174 times the clinical dose). Malformations included anomalies of the sternum, domed head, forepaw hyperextension and hindlimb malrotation, vertebral anomalies and delayed ossification of distal limb bones. A no observed adverse effect level (NOAEL) was established at 150 mcg/kg/day (87 times the clinical dose) in this study.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Latanoprostene bunod in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
Risk Summary
- There are no data on the presence of VYZULTA in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for VYZULTA, and any potential adverse effects on the breastfed infant from VYZULTA.
Pediatric Use
- Use in pediatric patients aged 16 years and younger is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use.
Geriatic Use
- No overall clinical differences in safety or effectiveness have been observed between elderly and other adult patients.
Gender
There is no FDA guidance on the use of Latanoprostene bunod with respect to specific gender populations.
Race
There is no FDA guidance on the use of Latanoprostene bunod with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Latanoprostene bunod in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Latanoprostene bunod in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Latanoprostene bunod in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Latanoprostene bunod in patients who are immunocompromised.
Administration and Monitoring
Administration
- If VYZULTA is to be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure, administer each drug product at least five (5) minutes apart.
- One drop in the affected eye(s) once daily in the evening
Monitoring
- A reduction in intraocular pressure is evidence of efficacy.
IV Compatibility
There is limited information regarding the compatibility of Latanoprostene bunod and IV administrations.
Overdosage
There is limited information regarding Latanoprostene bunod overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| Template:Px | |
Latanoprostene bunod
| |
| Systematic (IUPAC) name | |
| 4-Nitrooxybutyl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]hept-5-enoate | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Synonyms | BOL-303259-X |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Latanoprostene bunod is thought to lower intraocular pressure by increasing outflow of aqueous humor through both the trabecular meshwork and uveoscleral routes. Intraocular pressure is a major modifiable risk factor for glaucoma progression. Reduction of intraocular pressure reduces risk of glaucomatous visual field loss.
Structure
Pharmacodynamics
- Reduction of the intraocular pressure starts approximately 1 to 3 hours after the first administration with the maximum effect reached after 11-13 hours in eyes with elevated intraocular pressure.
Pharmacokinetics
Absorption
- The systemic exposure of latanoprostene bunod and its metabolites latanoprost acid and butanediol mononitrate were evaluated in one study with 22 healthy subjects after topical ocular administration of VYZULTA 0.024% once daily (one drop bilaterally in the morning) for 28 days. There were no quantifiable plasma concentrations of latanoprostene bunod (lower limit of quantitation, LLOQ, of 10.0 pg/mL) or butanediol mononitrate (LLOQ of 200 pg/mL) post dose on Day 1 and Day 28. The mean maximal plasma concentrations (Cmax) of latanoprost acid (LLOQ of 30 pg/mL) were 59.1 pg/mL and 51.1 pg/mL on Day 1 and Day 28, respectively. The mean time of maximal plasma concentration (Tmax) for latanoprost acid was approximately 5 min post administration on both Day 1 and Day 28.
Distribution
- There were no ocular distribution studies performed in humans.
Metabolism
- After topical ocular administration, latanoprostene bunod is rapidly metabolized in the eye to latanoprost acid (active moiety), an F2α prostaglandin analog, and butanediol mononitrate. After latanoprost acid reaches the systemic circulation, it is primarily metabolized by the liver to the 1,2-dinor and 1,2,3,4-tetranor metabolites via fatty acid β-oxidation.
- Butanediol mononitrate is metabolized to 1,4-butanediol and nitric oxide. The metabolite 1,4-butanediol is further oxidized to succinic acid and enters the tricarboxylic acid (TCA) cycle.
Elimination
- The elimination of latanoprost acid from human plasma is rapid as latanoprost acid plasma concentration dropped below the LLOQ (30 pg/mL) in the majority of subjects by 15 min following ocular administration of VYZULTA 0.024% in humans.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Latanoprostene bunod was not mutagenic in bacteria and did not induce micronuclei formation in the in vivo rat bone marrow micronucleus assay. Chromosomal aberrations were observed in vitro with human lymphocytes in the absence of metabolic activation.
- Latanoprostene bunod has not been tested for carcinogenic activity in long-term animal studies. Latanoprost acid is a main metabolite of latanoprostene bunod. Exposure of rats and mice to latanoprost acid, resulting from oral dosing with latanoprost in lifetime rodent bioassays, was not carcinogenic.
- Fertility studies have not been conducted with latanoprostene bunod. The potential to impact fertility can be partially characterized by exposure to latanoprost acid, a common metabolite of both latanoprostene bunod and latanoprost. Latanoprost acid has not been found to have any effect on male or female fertility in animal studies.
Animal Toxicology and/or Pharmacology
- A 9-month toxicology study administered topical ocular doses of latanoprostene bunod to one eye of cynomolgus monkeys: control (vehicle only), one drop of 0.024% bid, one drop of 0.04% bid and two drops of 0.04% per dose, bid. The systemic exposures are equivalent to 4.2-fold, 7.9-fold, and 13.5-fold the clinical dose, respectively, on a body surface area basis (assuming 100% absorption). Microscopic evaluation of the lungs after 9 months observed pleural/subpleural chronic fibrosis/inflammation in the 0.04% dose male groups, with increasing incidence and severity compared to controls. Lung toxicity was not observed at the 0.024% dose.
Clinical Studies
- In clinical studies up to 12 months duration, patients with open-angle glaucoma or ocular hypertension with average baseline intraocular pressures (IOPs) of 26.7 mmHg, the IOP-lowering effect of latanoprostene bunod ophthalmic solution 0.024% once daily (in the evening) was up to 7 to 9 mmHg.
How Supplied
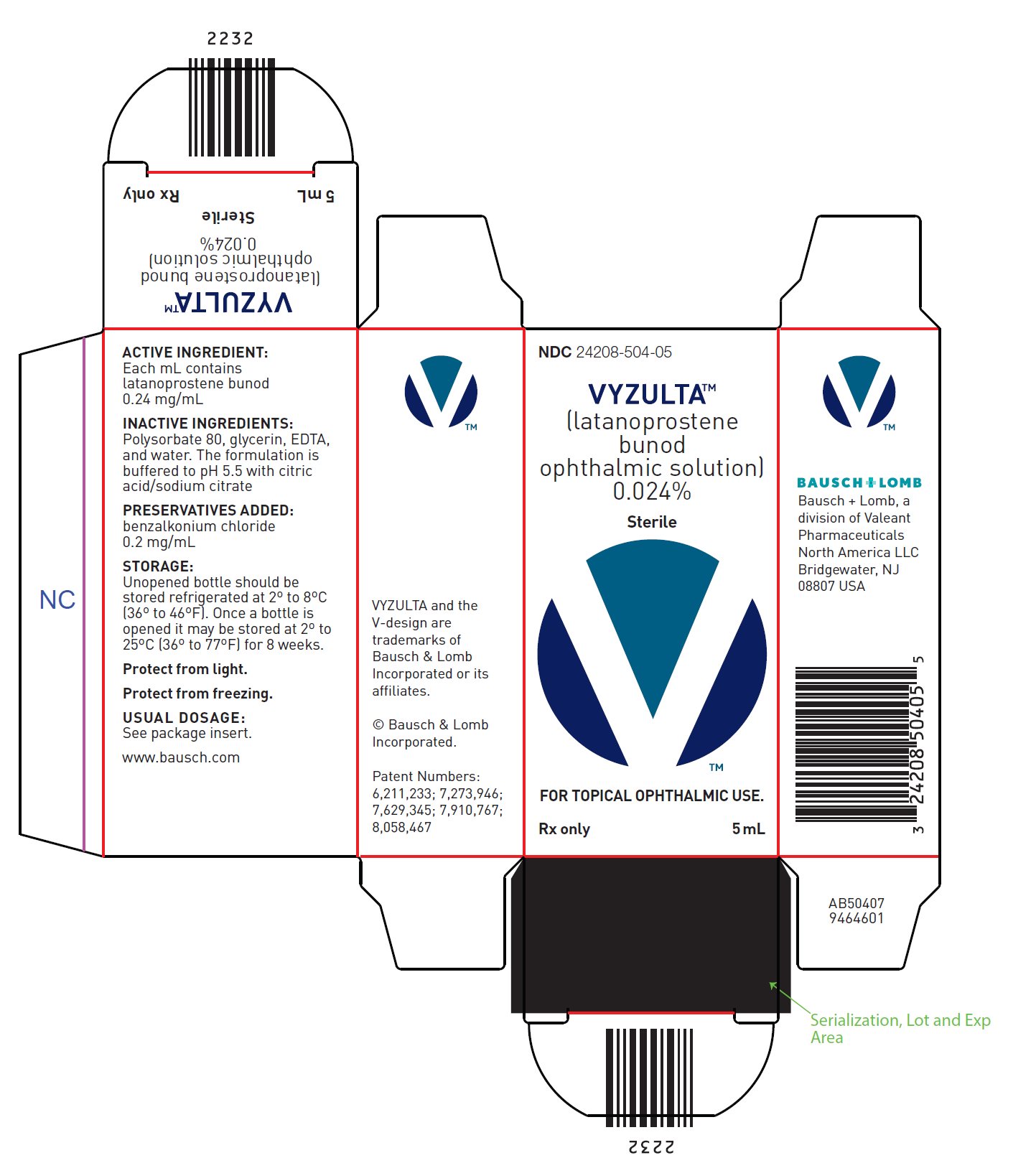
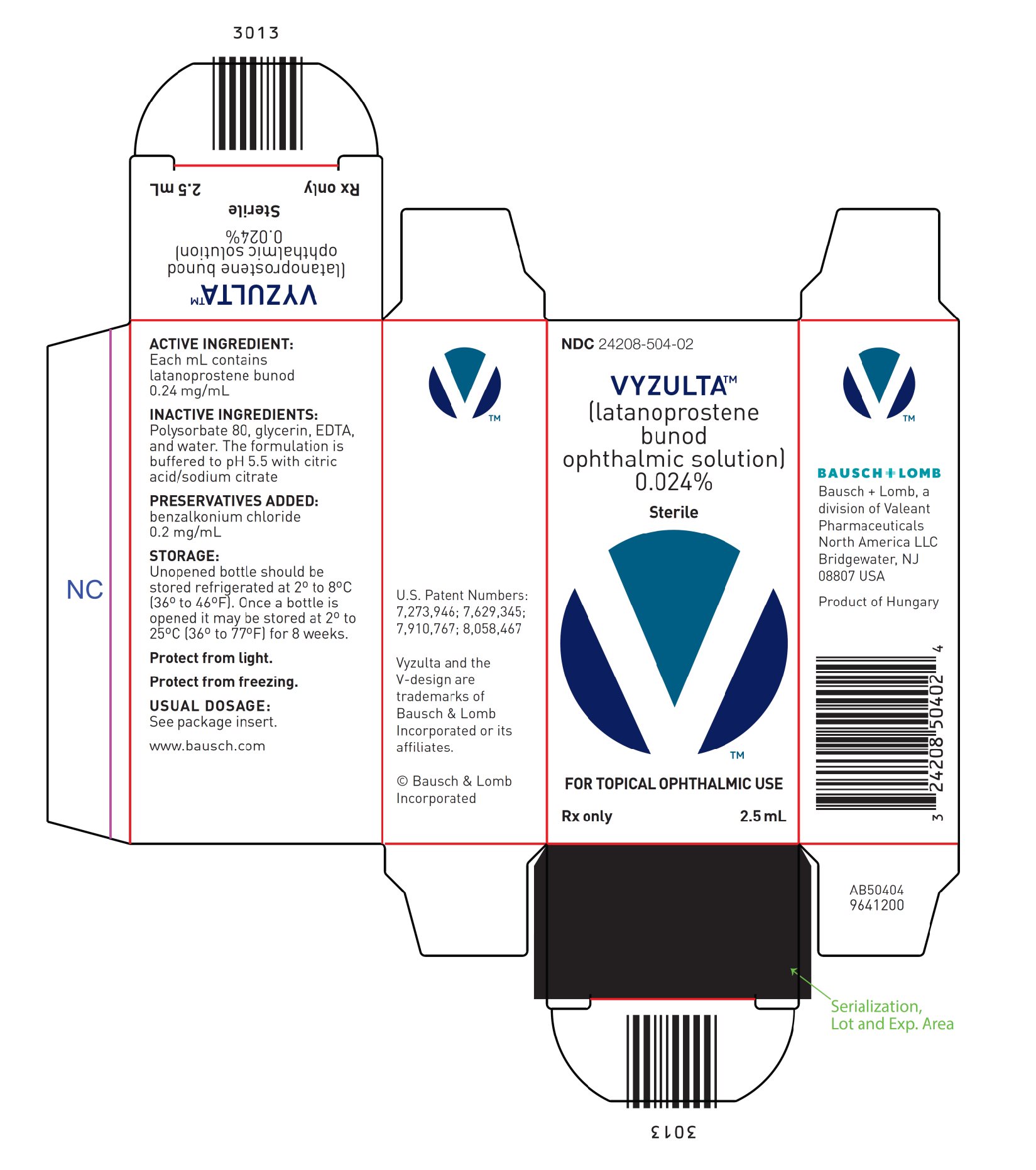
- Latanoprostene bunod ophthalmic solution, 0.024% is supplied in low density polyethylene bottles with dropper tips and turquoise caps in the following sizes:
- 2.5 mL fill in a 4 mL white container – NDC 24208-504-02
- 5 mL fill in a 7.5 mL natural container – NDC 24208-504-05
Storage
- Unopened bottle should be stored refrigerated at 2º to 8ºC (36º to 46ºF). Once a bottle is opened it may be stored at 2º to 25ºC (36º to 77ºF) for 8 weeks.
- During shipment, bottles may be maintained at temperatures up to 40°C (104°F) for a period not exceeding 14 days.
- Protect from light. Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Latanoprostene bunod |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Latanoprostene bunod |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Potential for Pigmentation
- Patients should be advised about the potential for increased brown pigmentation of the iris, which may be permanent. Patients should also be informed about the possibility of eyelid skin darkening, which is usually reversible after discontinuation of VYZULTA.
Potential for Eyelash Changes
- Patients should also be informed of the possibility of eyelash and vellus hair changes in the treated eye during treatment with VYZULTA. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
- Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
When to Seek Physician Advice
- Advise patients that if they develop a new ocular condition (e.g., trauma or infection), experience a sudden decrease in visual acuity, have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of VYZULTA.
Use with Contact Lenses
- Contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of VYZULTA.
Use with Other Ophthalmic Drugs
- If more than one topical ophthalmic drug is being used, the drugs should be administered with at least five (5) minutes between applications.
Precautions with Alcohol
Alcohol-Latanoprostene bunod interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Vyzulta
Look-Alike Drug Names
There is limited information regarding Latanoprostene bunod Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.