Fostamatinib: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= {{Sonya}} |genericName=generic name |aOrAn=a |drugClass=Acetylcholine release inhibitor, Adrenergic receptor agonist |indicationType=(t...") |
No edit summary |
||
| Line 299: | Line 299: | ||
|PK=(Description) | |PK=(Description) | ||
|nonClinToxic=(Description) | |nonClinToxic=(Description) | ||
|clinicalStudies= | |clinicalStudies= | ||
*Fostamitinib was studied in two placebo-controlled efficacy and safety studies (referred to as FIT-1 [NCT02076399] and FIT-2 [NCT02076412]), and in an open-label extension study referred to as FIT-3 (NCT 02077192). | |||
( | ''Randomized, Placebo-Controlled Studies'' | ||
*A total of 150 patients with persistent or chronic ITP, who had an insufficient response to previous treatment (which included corticosteroids, immunoglobulins, splenectomy, and/or a thrombopoietin receptor agonists) were enrolled in two identical, double-blind, placebo-controlled studies that were conducted in different countries. For each study, patients were randomized 2:1 to fostamatinib or placebo for 24 weeks; randomization was stratified with respect to prior splenectomy and severity of thrombocytopenia. Stable concurrent ITP therapy (glucocorticoids [< 20 mg prednisone equivalent per day], azathioprine, or danazol) was allowed, and rescue therapy was permitted, if needed. All patients initially received study drug at 100 mg twice daily (or matching placebo). Based on platelet count and tolerability, dose escalation to 150 mg twice daily (or matching placebo) was undertaken in 88% of patients at Week 4 or later. Patients who did not respond to treatment after 12 weeks, as well as patients who completed the 24-week double blind study, were eligible to enroll in open-label extension study (FIT-3). | |||
*Patients enrolled in the placebo-controlled studies had a median age of 54 years (range: 20 to 88), and the majority were female (61%) and were White (93%). Prior ITP treatments were varied, with the most common including corticosteroids (94%), immunoglobulins (53%), and thrombopoietin receptor agonists (TPO-RA) (48%). Most patients had chronic ITP (93%), with a median time since ITP diagnosis of 8.45 years, and 35% had undergone splenectomy. At baseline, the median platelet count was 16 × 10<sup>9</sup>/L (with almost half [45]%) less than 15 × 10<sup>9</sup>/L) and 47% were on stable ITP therapy. | |||
*In Study FIT-1, 76 patients were randomized; 51 to the fostamatinib group and 25 to the placebo group. In Study FIT-2, 74 patients were randomized; 50 to the fostamatinib group and 24 to the placebo group. The efficacy of fostamatinib was based on stable platelet response (at least 50 ×10<sup>9</sup>/L on at least 4 of the 6 visits between Weeks 14 to 24). Study outcomes for FIT-1 and FIT-2 are shown in Table 5. | |||
( | [[image:fostamatinibclinicalstudy1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
*In the FIT-1 and FIT-2 studies a total of 47 patients in the fostamatinib arm had received a prior TPO-RA treatment; among these patients, 8 patients (17%) achieved a stable response to fostamatinib. All 8 patients had previously discontinued TPO-RA due to loss of effect. Rescue medication was required by 30% and 45% of patients receiving fostamatinib or placebo, respectively. | |||
===== | *During the placebo-controlled studies, the incidence of bleeding occurred in 29% and 37% of patients in the fostamatinib and placebo arms, respectively. Moderate, severe and serious bleeding events are described in Table 6. All severe events led to hospitalizations. | ||
[[image:fostamatinibclinicalstudy2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
( | =====Extension Study===== | ||
*The FIT-3 trial is an open label extension study. Patients from FIT-1 and FIT-2 who completed 24 weeks of treatment, or who did not respond to treatment any time after 12 weeks, were eligible to enroll in this study. Patients remained blinded to their treatment assignment from the previous study (fostamatinib or placebo), so their starting dose in this study was based on their final platelet count. Patients designated as responders (defined as achievement of platelet count of at least 50 × 10<sup>9</sup>/L) at the time of roll over continued in the extension study at their current trial dose and regimen. Patients who entered the extension study as non-responders (defined as platelet count less than 50 × 10<sup>9</sup>/L) received fostamatinib 100 mg twice daily regardless of their dose and regimen in the prior study. | |||
*For the FIT-3 trial, 123 patients were enrolled, 44 patients previously randomized to placebo and 79 patients previously randomized to fostamatinib. Stable response in this study was prospectively defined as no 2 visits, at least 4 weeks apart, with a platelet count less than 50 × 10<sup>9</sup>/L, without an intervening visit with a platelet count of at least 50 × 10<sup>9</sup>/L (unrelated to rescue therapy), within a period of 12 weeks following initial achievement of the target platelet count. Sixty-one of the 123 subjects (50%) have discontinued from the study early. | |||
*In a prospectively defined analysis, the 44 subjects treated with placebo in the prior study were evaluated for stable response for fostamatinib. Ten of these subjects (23%) (including a single subject who was classified as a placebo responder in the prior study) met the criteria for stable response. | |||
*Among the subjects who achieved stable response in FIT-1, FIT-2 and FIT-3 trials, 18 subjects maintained the platelet count of at least 50 × 10<sup>9</sup>/L for 12 months or longer. | |||
|howSupplied= | |howSupplied= | ||
*Fostamatinib 100 mg tablets are round, biconvex, orange, film-coated tablets debossed with "100" on one side and "R" on the reverse side. | *Fostamatinib 100 mg tablets are round, biconvex, orange, film-coated tablets debossed with "100" on one side and "R" on the reverse side. | ||
Revision as of 18:18, 13 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fostamatinib is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fostamatinib in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Fostamatinib and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Fostamatinib
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
- Fostamitinib was studied in two placebo-controlled efficacy and safety studies (referred to as FIT-1 [NCT02076399] and FIT-2 [NCT02076412]), and in an open-label extension study referred to as FIT-3 (NCT 02077192).
Randomized, Placebo-Controlled Studies
- A total of 150 patients with persistent or chronic ITP, who had an insufficient response to previous treatment (which included corticosteroids, immunoglobulins, splenectomy, and/or a thrombopoietin receptor agonists) were enrolled in two identical, double-blind, placebo-controlled studies that were conducted in different countries. For each study, patients were randomized 2:1 to fostamatinib or placebo for 24 weeks; randomization was stratified with respect to prior splenectomy and severity of thrombocytopenia. Stable concurrent ITP therapy (glucocorticoids [< 20 mg prednisone equivalent per day], azathioprine, or danazol) was allowed, and rescue therapy was permitted, if needed. All patients initially received study drug at 100 mg twice daily (or matching placebo). Based on platelet count and tolerability, dose escalation to 150 mg twice daily (or matching placebo) was undertaken in 88% of patients at Week 4 or later. Patients who did not respond to treatment after 12 weeks, as well as patients who completed the 24-week double blind study, were eligible to enroll in open-label extension study (FIT-3).
- Patients enrolled in the placebo-controlled studies had a median age of 54 years (range: 20 to 88), and the majority were female (61%) and were White (93%). Prior ITP treatments were varied, with the most common including corticosteroids (94%), immunoglobulins (53%), and thrombopoietin receptor agonists (TPO-RA) (48%). Most patients had chronic ITP (93%), with a median time since ITP diagnosis of 8.45 years, and 35% had undergone splenectomy. At baseline, the median platelet count was 16 × 109/L (with almost half [45]%) less than 15 × 109/L) and 47% were on stable ITP therapy.
- In Study FIT-1, 76 patients were randomized; 51 to the fostamatinib group and 25 to the placebo group. In Study FIT-2, 74 patients were randomized; 50 to the fostamatinib group and 24 to the placebo group. The efficacy of fostamatinib was based on stable platelet response (at least 50 ×109/L on at least 4 of the 6 visits between Weeks 14 to 24). Study outcomes for FIT-1 and FIT-2 are shown in Table 5.

- In the FIT-1 and FIT-2 studies a total of 47 patients in the fostamatinib arm had received a prior TPO-RA treatment; among these patients, 8 patients (17%) achieved a stable response to fostamatinib. All 8 patients had previously discontinued TPO-RA due to loss of effect. Rescue medication was required by 30% and 45% of patients receiving fostamatinib or placebo, respectively.
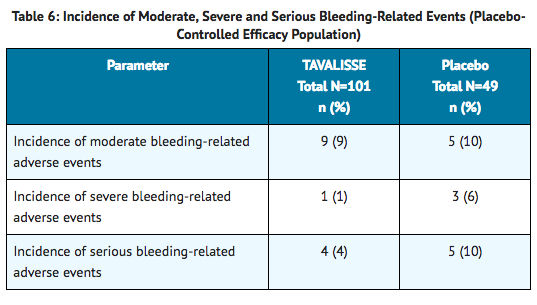
- During the placebo-controlled studies, the incidence of bleeding occurred in 29% and 37% of patients in the fostamatinib and placebo arms, respectively. Moderate, severe and serious bleeding events are described in Table 6. All severe events led to hospitalizations.
Extension Study
- The FIT-3 trial is an open label extension study. Patients from FIT-1 and FIT-2 who completed 24 weeks of treatment, or who did not respond to treatment any time after 12 weeks, were eligible to enroll in this study. Patients remained blinded to their treatment assignment from the previous study (fostamatinib or placebo), so their starting dose in this study was based on their final platelet count. Patients designated as responders (defined as achievement of platelet count of at least 50 × 109/L) at the time of roll over continued in the extension study at their current trial dose and regimen. Patients who entered the extension study as non-responders (defined as platelet count less than 50 × 109/L) received fostamatinib 100 mg twice daily regardless of their dose and regimen in the prior study.
- For the FIT-3 trial, 123 patients were enrolled, 44 patients previously randomized to placebo and 79 patients previously randomized to fostamatinib. Stable response in this study was prospectively defined as no 2 visits, at least 4 weeks apart, with a platelet count less than 50 × 109/L, without an intervening visit with a platelet count of at least 50 × 109/L (unrelated to rescue therapy), within a period of 12 weeks following initial achievement of the target platelet count. Sixty-one of the 123 subjects (50%) have discontinued from the study early.
- In a prospectively defined analysis, the 44 subjects treated with placebo in the prior study were evaluated for stable response for fostamatinib. Ten of these subjects (23%) (including a single subject who was classified as a placebo responder in the prior study) met the criteria for stable response.
- Among the subjects who achieved stable response in FIT-1, FIT-2 and FIT-3 trials, 18 subjects maintained the platelet count of at least 50 × 109/L for 12 months or longer.
How Supplied
- Fostamatinib 100 mg tablets are round, biconvex, orange, film-coated tablets debossed with "100" on one side and "R" on the reverse side.
- Fostamatinib 150 mg tablets are oval, biconvex, orange, film-coated tablets debossed with "150" on one side and "R" on the reverse side.

Storage
- Store at room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not remove desiccants.
Images
Drug Images
{{#ask: Page Name::Fostamatinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Fostamatinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling.
- Hypertension: Inform patients that periodic monitoring of their blood pressure is required, as high blood pressure has occurred in patients taking fostamatinib. Inform patients of the signs and symptoms of hypertension. Advise patients to undergo routine blood pressure monitoring and to contact their health care provider if blood pressure is elevated or if they experience signs or symptoms of hypertension.
- Hepatotoxicity: Inform patients that periodic monitoring of their liver enzymes is required, and any elevations (which may indicate liver injury) will be managed appropriately, including interruption, reduction, or discontinuation of fostamatinib.
- Diarrhea: Advise patients to use supportive care measures, and if diarrhea becomes severe, it may necessitate interruption, reduction, or discontinuation of fostamatinib.
- Neutropenia: Inform patients that monitoring of their complete blood counts is required, and a decrease in neutrophils may necessitate interruption, reduction, or discontinuation of fostamatinib.
- Advise patients to inform their healthcare providers of all their medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products.
- Embryo-Fetal Toxicity: Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the potential risk to a fetus.
- Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after receiving the last dose of fostamatinib.
- Lactation: Advise lactating women not to breastfeed during treatment with fostamatinib and for at least 1 month after the last dose.
- Inform patients that fostamatinib may be taken with or without food. In the case of a missed dose of fostamatinib, instruct patients to take their next dose at its regularly scheduled time.

Precautions with Alcohol
Alcohol-Fostamatinib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Tavalisse
Look-Alike Drug Names
There is limited information regarding Fostamatinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.