Tacrolimus (extended release): Difference between revisions
No edit summary |
No edit summary |
||
| Line 174: | Line 174: | ||
* The oral use of activated charcoal has been reported in treating acute overdoses, but experience has not been sufficient to warrant recommending its use. | * The oral use of activated charcoal has been reported in treating acute overdoses, but experience has not been sufficient to warrant recommending its use. | ||
* General supportive measures and treatment of specific symptoms should be followed in all cases of overdosage. | * General supportive measures and treatment of specific symptoms should be followed in all cases of overdosage. | ||
{{ | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 224: | Line 225: | ||
| legal_status = Rx-only | | legal_status = Rx-only | ||
| routes_of_administration = Topical, oral, [[Intravenous|iv]] | | routes_of_administration = Topical, oral, [[Intravenous|iv]] | ||
}} | }} | ||
Revision as of 19:15, 26 February 2017
{{DrugProjectFormSinglePage |authorTag=Shivani Chaparala M.B.B.S [1] |genericName=Tacrolimus (extended release) |aOrAn=a |drugClass=calcineurin-inhibitor immunosuppressant |indicationType=prophylaxis |indication=organ rejection in kidney transplant patients converted from tacrolimus immediate-release formulations in combination with other immunosuppressants |hasBlackBoxWarning=Yes |adverseReactions=(incidence ≥10%): diarrhea and blood creatinine increased |blackBoxWarningTitle=WARNING |blackBoxWarningBody=MALIGNANCIES AND SERIOUS INFECTIONS
- INCREASED RISK FOR DEVELOPING SERIOUS INFECTIONS AND MALIGNANCIES WITH ENVARSUS XR OR OTHER IMMUNOSUPPRESSANTS THAT MAY LEAD TO HOSPITALIZATION OR DEATH
|fdaLIADAdult=ENVARSUS XR is indicated for the prophylaxis of organ rejection in kidney transplant patients converted from tacrolimus immediate-release formulations, in combination with other immunosuppressants. Extended-release tablets: 0.75 mg, 1 mg, 4 mg.
Administration Instructions
- Take TACROLIMUS (EXTENDED RELEASE) on an empty stomach at the same time of the day, preferably in the morning (to ensure consistent and maximum possible drug exposure).
- Swallow TACROLIMUS (EXTENDED RELEASE) whole with fluid (preferably water); do not chew, divide, or crush the tablets.
- If a dose is missed, take it as soon as possible within 15 hours after missing the dose; beyond the 15-hour time frame, wait until the usual scheduled time to take the next regular daily dose. Do not double the next dose.
- Avoid eating grapefruit or drinking grapefruit juice or alcoholic beverage while taking TACROLIMUS (EXTENDED RELEASE).
- African-American patients, compared to Caucasian patients, may need to be titrated to higher TACROLIMUS (EXTENDED RELEASE) dosages to attain comparable trough concentrations.
Conversion from Tacrolimus Immediate-Release Formulations
- To convert from a tacrolimus immediate-release product to TACROLIMUS (EXTENDED RELEASE), administer an TACROLIMUS (EXTENDED RELEASE) once daily dose that is 80% of the total daily dose of the tacrolimus immediate-release product.
- Monitor tacrolimus whole blood trough concentrations and titrate TACROLIMUS (EXTENDED RELEASE) dosage to achieve target whole blood trough concentration ranges of 4 to 11 ng/mL.
Therapeutic Drug Monitoring
- Measure tacrolimus whole blood trough concentrations at least two times on separate days during the first week after initiation of dosing and after any change in dosage, after a change in co-administration of CYP3A inducers and/or inhibitors, or after a change in renal or hepatic function.
- When interpreting measured concentrations, consider that the time to achieve tacrolimus steady state is approximately 7 days after initiating or changing the TACROLIMUS (EXTENDED RELEASE) dose.
- Monitor tacrolimus whole blood trough concentrations using a validated assay [e.g., immunoassays or high-performance liquid chromatography with tandem mass spectrometric detection (HPLC/MS/MS)].
- The immunosuppressive activity of tacrolimus is mainly due to the parent drug rather than to its metabolites.
- Immunoassays may react with metabolites as well as the parent drug.
- Therefore, whole blood tacrolimus trough concentrations obtained with immunoassays may be numerically higher than concentrations obtained with an assay using HPLC/MS/MS.
- Comparison of the whole blood tacrolimus trough concentrations of patients to those described in the prescribing information and other published literature must be made with knowledge of the assay method(s) employed.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Tacrolimus (extended release) in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Tacrolimus (extended release) in adult patients. |fdaLIADPed=The safety and effectiveness of TACROLIMUS (EXTENDED RELEASE) in pediatric patients have not been established. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Tacrolimus (extended release) in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Tacrolimus (extended release) in pediatric patients. |contraindications=* TACROLIMUS (EXTENDED RELEASE) is contraindicated in patients with known hypersensitivity to tacrolimus. |warnings===== Lymphoma and Other Malignancies ====
- Immunosuppressants, including TACROLIMUS (EXTENDED RELEASE), increase the risk of developing lymphomas and other malignancies, particularly of the skin.
- The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
- Examine patients for skin changes and advise to avoid or limit exposure to sunlight and UV light.
- Post-transplant lymphoproliferative disorder (PTLD), associated with Epstein-Barr Virus (EBV), has been reported in immunosuppressed organ transplant patients.
- The risk of PTLD appears greatest in those individuals who are EBV seronegative. Monitor EBV serology during treatment.
Serious Infections
- Immunosuppressants, including TACROLIMUS (EXTENDED RELEASE), increase the risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections.
- These infections may lead to serious, including fatal, outcomes. Serious viral infections reported include:
- Polyomavirus-associated nephropathy (especially due to BK virus infection),
- JC virus-associated progressive multifocal leukoencephalopathy (PML), and
- Cytomegalovirus (CMV) infections: CMV seronegative transplant patients who receive an organ from a CMV seropositive donor are at highest risk of CMV viremia and CMV disease.
- Monitor for the development of infection and adjust the immunosuppressive regimen to balance the risk of rejection with the risk of infection.
Graft Rejection and Other Serious Adverse Reactions due to Medication Errors
- Medication errors, including substitution and dispensing errors, between tacrolimus immediate-release products and tacrolimus extended-release products were reported outside the U.S.
- This led to serious adverse reactions, including graft rejection, or other adverse reactions due to under- or over-exposure to tacrolimus.
- TACROLIMUS (EXTENDED RELEASE) is not interchangeable or substitutable with tacrolimus immediate-release products or other tacrolimus extended-release products.
- Instruct patients and caregivers to recognize the appearance of TACROLIMUS (EXTENDED RELEASE) tablet.
New Onset Diabetes After Transplant
- TACROLIMUS (EXTENDED RELEASE) caused new onset diabetes after transplant (NODAT) in kidney transplant patients, which may be reversible in some patients.
- African-American and Hispanic kidney transplant patients are at an increased risk.
- Monitor blood glucose concentrations and treat appropriately.
Nephrotoxicity due to TACROLIMUS (EXTENDED RELEASE) and Drug Interactions
- TACROLIMUS (EXTENDED RELEASE), like other calcineurin-inhibitors, can cause acute or chronic nephrotoxicity.
- Consider dosage reduction in patients with elevated serum creatinine and tacrolimus whole blood trough concentrations greater than the recommended range.
- The risk for nephrotoxicity may increase when TACROLIMUS (EXTENDED RELEASE) is concomitantly administered with CYP3A inhibitors (by increasing tacrolimus whole blood concentrations) or drugs associated with nephrotoxicity (e.g., aminoglycosides, ganciclovir, amphotericin B, cisplatin, nucleotide reverse transcriptase inhibitors, protease inhibitors).
- Monitor renal function and consider dosage reduction if nephrotoxicity occurs.
Neurotoxicity
- TACROLIMUS (EXTENDED RELEASE) may cause a spectrum of neurotoxicities.
- The most severe neurotoxicities include posterior reversible encephalopathy syndrome (PRES), delirium, seizure, and coma; others include tremors, paresthesias, headache, mental status changes, and changes in motor and sensory functions.
- As symptoms may be associated with tacrolimus whole blood trough concentrations at or above the recommended range, monitor for neurologic symptoms and consider dosage reduction or discontinuation of TACROLIMUS (EXTENDED RELEASE) if neurotoxicity occurs.
Hyperkalemia
- Mild to severe hyperkalemia, which may require treatment, has been reported with tacrolimus including TACROLIMUS (EXTENDED RELEASE).
- Concomitant use of agents associated with hyperkalemia (e.g., potassium-sparing diuretics, ACE inhibitors, angiotensin receptor blockers) may increase the risk for hyperkalemia.
- Monitor serum potassium levels periodically during treatment.
Hypertension
- Hypertension is a common adverse reaction of TACROLIMUS (EXTENDED RELEASE) therapy and may require antihypertensive therapy.
- Some antihypertensive drugs can increase the risk for hyperkalemia.
- Calcium-channel blocking agents may increase tacrolimus blood concentrations and require dosage reduction of TACROLIMUS (EXTENDED RELEASE).
Risk of Rejection with Strong CYP3A Inducers and Risk of Serious Adverse Reactions with Strong CYP3A Inhibitors
- The concomitant use of strong CYP3A inducers may increase the metabolism of tacrolimus, leading to lower whole blood trough concentrations and greater risk of rejection.
- In contrast, the concomitant use of strong CYP3A inhibitors may decrease the metabolism of tacrolimus, leading to higher whole blood trough concentrations and greater risk of serious adverse reactions (e.g., neurotoxicity, QT prolongation).
- Therefore, adjust TACROLIMUS (EXTENDED RELEASE) dose and monitor tacrolimus whole blood trough concentrations when coadministering TACROLIMUS (EXTENDED RELEASE) with strong CYP3A inhibitors (e.g., telaprevir, boceprevir, ritonavir, ketoconazole, itraconazole, voriconazole, clarithromycin) or strong CYP3A inducers (e.g., rifampin, rifabutin).
QT Prolongation
- TACROLIMUS (EXTENDED RELEASE) may prolong the QT/QTc interval and cause Torsade de Pointes.
- Avoid TACROLIMUS (EXTENDED RELEASE) in patients with congenital long QT syndrome.
- Consider obtaining electrocardiograms and monitoring electrolytes (magnesium, potassium, calcium) periodically during treatment in patients with congestive heart failure, bradyarrhythmias, those taking certain antiarrhythmic medications or other products that lead to QT prolongation, and those with electrolyte disturbances (e.g., hypokalemia, hypocalcemia, or hypomagnesemia).
- Consider obtaining electrocardiograms and monitoring electrolytes (magnesium, potassium, calcium) periodically during treatment in patients with congestive heart failure, bradyarrhythmias, those taking certain antiarrhythmic medications or other products that lead to QT prolongation, and those with electrolyte disturbances (e.g., hypokalemia, hypocalcemia, or hypomagnesemia).
- When coadministering TACROLIMUS (EXTENDED RELEASE) with other substrates and/or inhibitors of CYP3A, a reduction in ENVARSUS XR dosage, monitoring of tacrolimus whole blood concentrations, and monitoring for QT prolongation is recommended.
Immunizations
- Whenever possible, administer the complete complement of vaccines before transplantation and treatment with TACROLIMUS (EXTENDED RELEASE).
- Avoid the use of live attenuated vaccines during treatment with TACROLIMUS (EXTENDED RELEASE) (e.g., intranasal influenza, measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid vaccines).
- Inactivated vaccines noted to be safe for administration after transplantation may not be sufficiently immunogenic during treatment with TACROLIMUS (EXTENDED RELEASE).
Pure Red Cell Aplasia
- Cases of pure red cell aplasia (PRCA) have been reported in patients treated with tacrolimus.
- All of these patients reported risk factors for PRCA such as parvovirus B19 infection, underlying disease, or concomitant medications associated with PRCA.
- A mechanism for tacrolimus-induced PRCA has not been elucidated. If PRCA is diagnosed, consider discontinuation of TACROLIMUS (EXTENDED RELEASE).
|clinicalTrials=* Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
- In addition, the clinical studies were not designed to establish comparative differences across study arms with regards to the adverse reactions discussed below.
- In an open label, randomized, multinational conversion study, stable kidney transplant patients on a tacrolimus immediate-release product and concomitant immunosuppressants were randomized to treatment with TACROLIMUS (EXTENDED RELEASE) (N=162) or to continued treatment on the tacrolimus immediate-release product (N=162) and treated for a duration of 12 months.
- The proportion of patients who discontinued treatment due to adverse reactions was 7.4% and 1.2% in the ENVARSUS XR and tacrolimus immediate-release treatment groups, respectively, through 12 months of treatment.
- The most common adverse reactions leading to discontinuation of study drug in the TACROLIMUS (EXTENDED RELEASE) treatment group was cardiac arrest (2 events).
Infections
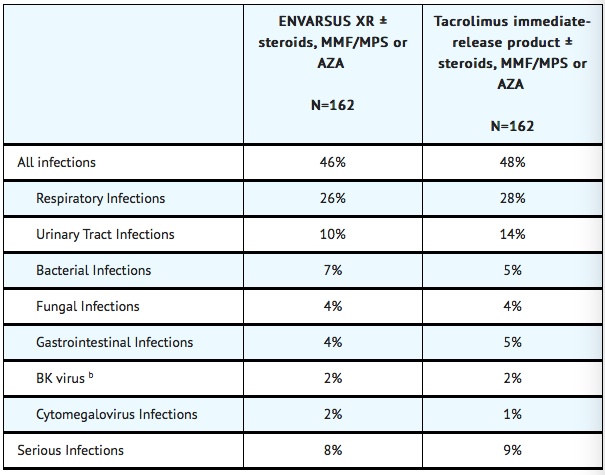
- The overall incidence of infections, serious infections, and infections with identified etiology reported in stable kidney transplant recipients treated with TACROLIMUS (EXTENDED RELEASE) or tacrolimus immediate-release product are shown in TABLE 1. Percentage of Stable Patients with Infections Through One Year Post- Treatment in the Conversion Study (a)
(a) The stable kidney transplant study was not designed to support comparative claims of ENVARSUS XR compared to tacrolimus immediate-release product for the adverse reactions reported in this table.
(b) BK virus associated nephropathy (BKVAN) occurred in 1.2% (2/162) and 0.6% (1/162) in the ENVARSUS XR and tacrolimus immediate-release treatment groups, respectively.
New Onset Diabetes After Transplantation (NODAT)
- New onset diabetes after transplantation (NODAT) was defined by the composite occurrence of fasting plasma glucose values ≥126 mg/dL, 2-hour postprandial plasma glucose of at least 200 mg/dL (in oral glucose tolerance test) on 2 or more consecutive occasions post baseline, insulin requirement for ≥31 days, an oral hypoglycemic agent use ≥31 days, or HbA1c ≥6.5% (at least 3 months after randomization) among kidney transplant patients with no medical history of diabetes.
- The incidence of NODAT for the stable kidney transplant study through one year post-transplant is summarized in TABLE 2 below.
Common Adverse Reactions
- The incidence of adverse reactions that occurred in ≥5% of ENVARSUS XR-treated patients compared to tacrolimus immediate-release product through one year of treatment in the conversion study is shown by treatment group in TABLE 3.
|postmarketing=* The following adverse reactions have been reported from marketing experience with tacrolimus in the U.S. and outside the U.S.
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Agranulocytosis, decreased blood fibrinogen, disseminated intravascular coagulation, hemolytic anemia, hemolytic uremic syndrome, pancytopenia, prolonged activated partial thromboplastin time, pure red cell aplasia, thrombocytopenic purpura, thrombotic thrombocytopenia purpura.
- Cardiac Disorders: Atrial fibrillation, atrial flutter, cardiac arrhythmia, cardiac arrest, electrocardiogram T wave abnormal, flushing, myocardial hypertrophy, myocardial infarction, myocardial ischaemia, pericardial effusion, QT prolongation, supraventricular extrasystoles, supraventricular tachycardia, Torsade de Pointes, deep limb venous thrombosis, ventricular fibrillation.
- Ear Disorders: Hearing loss including deafness.
- Eye Disorders: Blindness, photophobia, optic atrophy.
- Gastrointestinal Disorders: Colitis, dysphagia, gastrointestinal perforation, impaired gastric emptying, intestinal obstruction, mouth ulceration, peritonitis, stomach ulcer.
- Hepatobiliary Disorders: Bile duct stenosis, cholangitis, cirrhosis, fatty liver, hepatic cytolysis, hepatic failure, hepatic necrosis, hepatic steatosis, jaundice, hemorrhagic pancreatitis, necrotizing pancreatitis, venoocclusive liver disease.
- Hypersensitivity Reactions: Hypersensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria.
- Immune System Disorders: Graft versus host disease (acute and chronic).
- Metabolism and Nutrition Disorders: Glycosuria, increased amylase, pancreatitis.
- Musculoskeletal and Connective Tissue Disorders: Myalgia, polyarthritis, rhabdomyolysis.
- Neoplasms: Lymphoma including EBV-associated lymphoproliferative disorder, PTLD, leukemia.
- Nervous System Disorders: Carpal tunnel syndrome, cerebral infarction, coma, dysarthria, flaccid paralysis, hemiparesis, mental disorder, mutism, nerve compression, posterior reversible encephalopathy syndrome (PRES), progressive multifocal leukoencephalopathy (PML) sometimes fatal, quadriplegia, speech disorder, status epilepticus, syncope.
- Renal and Urinary Disorder: Acute renal failure, hemorrhagic cystitis, hemolytic uremic syndrome, micturition disorder.
- Respiratory, Thoracic and Mediastinal Disorders: Acute respiratory distress syndrome, interstitial lung disease, lung infiltration, pulmonary hypertension, respiratory distress, respiratory failure.
- Skin and Subcutaneous Tissue Disorders: Hyperpigmentation, photosensitivity.
|drugInteractions===== Mycophenolic Acid ====
- When ENVARSUS XR is prescribed with a given dose of mycophenolic acid (MPA) product, exposure to MPA is higher with ENVARSUS XR coadministration than with cyclosporine coadministration because cyclosporine interrupts the enterohepatic recirculation of MPA while tacrolimus does not.
- Monitor for MPA associated adverse reactions and reduce the dose of concomitantly administered mycophenolic acid products as needed.
Effects of Other Drugs/Substances on ENVARSUS XR
|FDAPregCat=C |useInPregnancyFDA=* There are no adequate and well-controlled studies in pregnant women.
- Tacrolimus is transferred across the placenta.
- The use of tacrolimus during pregnancy in humans has been associated with neonatal hyperkalemia and renal dysfunction.
- Tacrolimus given orally to pregnant rabbits at 0.7 times the maximum clinical dose and pregnant rats at 1.1 times the maximum clinical dose was associated with an increased incidence of fetal death in utero, fetal malformations (cardiovascular, skeletal, omphalocele, and gallbladder agenesis) and maternal toxicity.
- ENVARSUS XR should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
- In pregnant rabbits, tacrolimus at oral doses of 0.32 and 1.0 mg/kg (0.7 and 2.3 times the maximum clinical dose based on body surface area, respectively) was associated with maternal toxicity as well as an increased incidence of abortions.
- At the 1 mg/kg dose, fetal rabbits showed an increased incidence of malformations (ventricular hypoplasia, interventricular septal defect, bulbous aortic arch, stenosis of ductus arteriosis, interrupted ossification of vertebral arch, vertebral and rib malformations, omphalocele, and gallbladder agenesis) and developmental variations.
- In pregnant rats, tacrolimus at oral doses of 3.2 mg/kg (3.7 times the maximum clinical dose) was associated with maternal toxicity, an increase in late resorptions, decreased numbers of live births, and decreased pup weight and viability.
- Tacrolimus, given orally to pregnant rats after organogenesis and during lactation at 1.0 and 3.2 mg/kg (1.2 and 3.7 times the maximum recommended clinical dose, respectively) was associated with reduced pup weights and pup viability (3.2 mg/kg only); among the high dose pups that died early, an increased incidence of kidney hydronephrosis was observed.
|useInNursing=* Tacrolimus is present in breast milk. Because of the potential for serious adverse drug reactions in nursing infants from ENVARSUS XR, a decision should be made whether to discontinue nursing or to discontinue ENVARSUS XR, taking into account the importance of drug to the mother. |useInPed=* The safety and effectiveness of ENVARSUS XR in pediatric patients have not been established. |useInGeri=* Clinical studies of ENVARSUS XR did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In the stable kidney transplant study, there were 17 patients 65 years of age and older, and no patients were over 75 years.
- Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
- In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
|useInRace=* African-American patients may need to be titrated to higher ENVARSUS XR dosages to attain comparable trough concentrations compared to Caucasian patients. |useInRenalImpair=* The pharmacokinetics of tacrolimus in patients with renal impairment was similar to that in healthy subjects with normal renal function.
- However, due to its potential for nephrotoxicity, monitoring of renal function in patients with renal impairment is recommended; tacrolimus dosage should be reduced if indicated.
|useInHepaticImpair=* The mean clearance of tacrolimus was substantially lower in patients with severe hepatic impairment (mean Child-Pugh score: >10) compared to healthy subjects with normal hepatic function.
- With greater tacrolimus whole blood trough concentrations in patients with severe hepatic impairment, there is a greater risk of adverse reactions and dosage reduction is recommended.
- For patients with moderate hepatic impairment, monitor tacrolimus whole blood trough concentrations.
- For patients with mild hepatic impairment, no dosage adjustments are needed.
|administration=* Take TACROLIMUS (EXTENDED RELEASE) on an empty stomach at the same time of the day, preferably in the morning (to ensure consistent and maximum possible drug exposure).
- Swallow TACROLIMUS (EXTENDED RELEASE) whole with fluid (preferably water); do not chew, divide, or crush the tablets.
- If a dose is missed, take it as soon as possible within 15 hours after missing the dose; beyond the 15-hour time frame, wait until the usual scheduled time to take the next regular daily dose. Do not double the next dose.
- Avoid eating grapefruit or drinking grapefruit juice or alcoholic beverage while taking TACROLIMUS (EXTENDED RELEASE).
- African-American patients, compared to Caucasian patients, may need to be titrated to higher TACROLIMUS (EXTENDED RELEASE) dosages to attain comparable trough concentrations.
|monitoring=* Measure tacrolimus whole blood trough concentrations at least two times on separate days during the first week after initiation of dosing and after any change in dosage, after a change in co-administration of CYP3A inducers and/or inhibitors, or after a change in renal or hepatic function.
- When interpreting measured concentrations, consider that the time to achieve tacrolimus steady state is approximately 7 days after initiating or changing the TACROLIMUS (EXTENDED RELEASE) dose.
- Monitor tacrolimus whole blood trough concentrations using a validated assay [e.g., immunoassays or high-performance liquid chromatography with tandem mass spectrometric detection (HPLC/MS/MS)].
- The immunosuppressive activity of tacrolimus is mainly due to the parent drug rather than to its metabolites.
- Immunoassays may react with metabolites as well as the parent drug.
- Therefore, whole blood tacrolimus trough concentrations obtained with immunoassays may be numerically higher than concentrations obtained with an assay using HPLC/MS/MS.
- Comparison of the whole blood tacrolimus trough concentrations of patients to those described in the prescribing information and other published literature must be made with knowledge of the assay method(s) employed.
|overdose=* Postmarketing cases of overdose with tacrolimus have been reported. Overdosage adverse reactions included:
- Nervous system disorders (tremor, headache, confusional state, balance disorders, encephalopathy, lethargy and somnolence).
- Gastrointestinal disturbances (nausea, vomiting, and diarrhea).
- Abnormal renal function (increased blood urea nitrogen and elevated serum creatinine).
- Urticaria.
- Hypertension.
- Peripheral edema, and
- Infections [one fatal postmarketing case of bilateral pneumopathy and CMV infection was attributed to tacrolimus (extended-release capsules) overdose].
- Based on the poor aqueous solubility and extensive erythrocyte and plasma protein binding, it is anticipated that tacrolimus is not dialyzable to any significant extent; there is no experience with charcoal hemoperfusion.
- The oral use of activated charcoal has been reported in treating acute overdoses, but experience has not been sufficient to warrant recommending its use.
- General supportive measures and treatment of specific symptoms should be followed in all cases of overdosage.
{{ |drugBox=
| Template:Px | |
| Template:Px | |
Tacrolimus (extended release)
| |
| Systematic (IUPAC) name | |
| 3S-[3R*[E(1S*,3S*,4S*)] ,4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR* | |
| Identifiers | |
| CAS number | |
| ATC code | D11 L04AD02 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 804.018 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 24% (5–67%), less after eating food rich in fat |
| Protein binding | ≥98.8% |
| Metabolism | Hepatic CYP3A4, CYP3A5 |
| Half life | 11.3 h for transplant patients (range 3.5–40.6 h) |
| Excretion | Mostly faecal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Topical, oral, iv |