Oxymorphone (injection): Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= <!--Overview--> |genericName= |aOrAn= a |drugClass= |indication= |hasBlackBoxWarning= Yes |adverseReactions= <!--Bla...") |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{AV}} | ||
<!--Overview--> | <!--Overview--> | ||
|genericName= | |genericName=Oxymorphone (injection) | ||
| Line 44: | Line 44: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
*OPANA Injection is indicated for the relief of moderate to severe pain. It is also indicated for preoperative medication, for support of anesthesia, for obstetrical analgesia, and for relief of anxiety in patients with dyspnea associated with pulmonary edema secondary to acute left ventricular dysfunction. | |||
====Dosage==== | |||
*OPANA Injection is an opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine and other opioids. | |||
*OPANA Injection, like morphine and other opioids used in analgesia, can be abused and is subject to criminal diversion. | |||
*Selection of patients for treatment with OPANA Injection should be governed by the same principles that apply to the use of similar opioid analgesics (see INDICATIONS AND USAGE). Physicians should individualize treatment in every case (see DOSAGE AND ADMINISTRATION), using non-opioid analgesics, prn opioids and/or combination products, and chronic opioid therapy in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Healthcare Research and Quality, and the American Pain Society. | |||
*As with any opioid drug product, it is necessary to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. In the selection of the initial dose of OPANA Injection, attention should be given to the following: | |||
:*The total daily dose, potency and specific characteristics of the opioid the patient has been taking previously; | |||
:*The relative potency estimate used to calculate the equivalent oxymorphone dose needed; | |||
:*The patient’s degree of opioid tolerance; | |||
:*The age, general condition, and medical status of the patient; | |||
:*Concurrent non-opioid analgesic and other medications; | |||
:*The type and severity of the patient's pain; | |||
:*The balance between pain control and adverse experiences. | |||
:*Risk factors for abuse, addiction or diversion, including a prior history of abuse, addiction or diversion. | |||
*The following dosing recommendations, therefore, can only be considered as suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient. | |||
*Initiation of Therapy | |||
:*Subcutaneous or intramuscular administration: initially 1 mg to 1.5 mg, repeated every 4 to 6 hours as needed. Intravenous: 0.5 mg initially. In non debilitated patients the dose can be cautiously increased until satisfactory pain relief is obtained. For analgesia during labor 0.5 mg to 1 mg intramuscularly is recommended. | |||
*Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. | |||
:*Conversion from Oral OPANA to OPANA Injection | |||
:*Given the absolute oral bioavailability of approximately 10%, patients receiving oral OPANA may be converted to OPANA Injection by administering one-tenth the patient’s total daily oral oxymorphone dose as OPANA Injectable in four or six equally divided doses (e.g., total daily Oral dose/ [10 x 4]). For example, approximately 1 mg of OPANA Injectable IM every 6 hours (4 mg total IM dose) may be required to provide pain relief equivalent to a total daily dose of 40 mg oral OPANA. The dose can be titrated to optimal pain relief or combined with acetaminophen/NSAIDs for optimal pain relief. Due to patient variability with regard to opioid analgesic response, upon conversion patients should be closely monitored to ensure adequate analgesia and to minimize side effects. | |||
*Individualization of Dose | |||
:*Once therapy is initiated, pain relief and other opioid effects should be frequently assessed. Patients should be titrated to adequate pain relief (generally mild or no pain). Patients who experience breakthrough pain may require dosage adjustment or non-opioid therapy such as acetaminophen or NSAIDs. | |||
:*If signs of excessive opioid-related adverse experiences are observed, the next dose may be reduced. Dose adjustments should be made to obtain an appropriate balance between pain relief and opioid-related adverse experiences. If significant adverse events occur before the therapeutic goal of mild or no pain is achieved, the events should be treated aggressively. Once adverse events are under control, upward titration should continue to an acceptable level of pain control. | |||
:*During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the health-care team, the patient and the caregiver/family. Patients and family members should be advised of the potential common side effects to decrease fear of the use of opioids and promote their optimal use. | |||
*Patients with Hepatic Impairment | |||
:*The effects of OPANA Injection on hepatic impairment have not been studied. However, OPANA Injection is contraindicated in patients with moderate and severe hepatic dysfunction. OPANA Injection should be used with caution in patients with mild hepatic impairment. These patients with mild hepatic impairment should be started with the lowest dose and titrated slowly while carefully monitoring side effects (see CLINICAL PHARMACOLOGY, CONTRAINDICATIONS, and PRECAUTIONS). | |||
*Patients with Renal Impairment | |||
:*The effects of OPANA Injection on renal impairment have not been studied. However, there are 57% and 65% increases in oxymorphone bioavailability in patients with moderate to severe renal impairment, respectively, treated with OPANA ER (see CLINICAL PHARMACOLOGY and PRECAUTIONS). Accordingly, OPANA Injection should be administered cautiously and in reduced dosages to patients with creatinine clearance rate less than 50 mL/min. | |||
*Use with CNS depressants | |||
:*OPANA Injection, like all opioid analgesics, should be started at 1/3 to 1/2 of the usual dose in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol because respiratory depression, hypotension and profound sedation or coma may result. No specific interaction between oxymorphone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate (see PRECAUTIONS: GENERAL andPRECAUTIONS: DRUG-DRUG INTERACTIONS) | |||
*Geriatrics | |||
:*Caution should be exercised in the selection of the starting dose of OPANA Injection for an elderly patient starting at the low end of the dosing range. | |||
*Maintenance of Therapy | |||
:*OPANA Injection is intended as an opioid analgesic for the management of moderate to severe pain where the use of an opioid analgesic is appropriate. During therapy, continual re-evaluation of the patient receiving OPANA Injection is important, with special attention to the maintenance of pain control and the relative incidence of side effects associated with therapy. If the level of pain increases, effort should be made to identify the source of increased pain, while adjusting the dose and/or using adjuvant analgesics such as acetaminophen or NSAIDs. | |||
*Cessation of Therapy | |||
:*When the patient no longer requires therapy with OPANA Injection, doses should be tapered gradually to prevent signs and symptoms of withdrawal in the physically dependent patient. | |||
| Line 134: | Line 194: | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
Revision as of 20:01, 20 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Oxymorphone (injection) is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- OPANA Injection is indicated for the relief of moderate to severe pain. It is also indicated for preoperative medication, for support of anesthesia, for obstetrical analgesia, and for relief of anxiety in patients with dyspnea associated with pulmonary edema secondary to acute left ventricular dysfunction.
Dosage
- OPANA Injection is an opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine and other opioids.
- OPANA Injection, like morphine and other opioids used in analgesia, can be abused and is subject to criminal diversion.
- Selection of patients for treatment with OPANA Injection should be governed by the same principles that apply to the use of similar opioid analgesics (see INDICATIONS AND USAGE). Physicians should individualize treatment in every case (see DOSAGE AND ADMINISTRATION), using non-opioid analgesics, prn opioids and/or combination products, and chronic opioid therapy in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Healthcare Research and Quality, and the American Pain Society.
- As with any opioid drug product, it is necessary to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. In the selection of the initial dose of OPANA Injection, attention should be given to the following:
- The total daily dose, potency and specific characteristics of the opioid the patient has been taking previously;
- The relative potency estimate used to calculate the equivalent oxymorphone dose needed;
- The patient’s degree of opioid tolerance;
- The age, general condition, and medical status of the patient;
- Concurrent non-opioid analgesic and other medications;
- The type and severity of the patient's pain;
- The balance between pain control and adverse experiences.
- Risk factors for abuse, addiction or diversion, including a prior history of abuse, addiction or diversion.
- The following dosing recommendations, therefore, can only be considered as suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient.
- Initiation of Therapy
- Subcutaneous or intramuscular administration: initially 1 mg to 1.5 mg, repeated every 4 to 6 hours as needed. Intravenous: 0.5 mg initially. In non debilitated patients the dose can be cautiously increased until satisfactory pain relief is obtained. For analgesia during labor 0.5 mg to 1 mg intramuscularly is recommended.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
- Conversion from Oral OPANA to OPANA Injection
- Given the absolute oral bioavailability of approximately 10%, patients receiving oral OPANA may be converted to OPANA Injection by administering one-tenth the patient’s total daily oral oxymorphone dose as OPANA Injectable in four or six equally divided doses (e.g., total daily Oral dose/ [10 x 4]). For example, approximately 1 mg of OPANA Injectable IM every 6 hours (4 mg total IM dose) may be required to provide pain relief equivalent to a total daily dose of 40 mg oral OPANA. The dose can be titrated to optimal pain relief or combined with acetaminophen/NSAIDs for optimal pain relief. Due to patient variability with regard to opioid analgesic response, upon conversion patients should be closely monitored to ensure adequate analgesia and to minimize side effects.
- Individualization of Dose
- Once therapy is initiated, pain relief and other opioid effects should be frequently assessed. Patients should be titrated to adequate pain relief (generally mild or no pain). Patients who experience breakthrough pain may require dosage adjustment or non-opioid therapy such as acetaminophen or NSAIDs.
- If signs of excessive opioid-related adverse experiences are observed, the next dose may be reduced. Dose adjustments should be made to obtain an appropriate balance between pain relief and opioid-related adverse experiences. If significant adverse events occur before the therapeutic goal of mild or no pain is achieved, the events should be treated aggressively. Once adverse events are under control, upward titration should continue to an acceptable level of pain control.
- During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the health-care team, the patient and the caregiver/family. Patients and family members should be advised of the potential common side effects to decrease fear of the use of opioids and promote their optimal use.
- Patients with Hepatic Impairment
- The effects of OPANA Injection on hepatic impairment have not been studied. However, OPANA Injection is contraindicated in patients with moderate and severe hepatic dysfunction. OPANA Injection should be used with caution in patients with mild hepatic impairment. These patients with mild hepatic impairment should be started with the lowest dose and titrated slowly while carefully monitoring side effects (see CLINICAL PHARMACOLOGY, CONTRAINDICATIONS, and PRECAUTIONS).
- Patients with Renal Impairment
- The effects of OPANA Injection on renal impairment have not been studied. However, there are 57% and 65% increases in oxymorphone bioavailability in patients with moderate to severe renal impairment, respectively, treated with OPANA ER (see CLINICAL PHARMACOLOGY and PRECAUTIONS). Accordingly, OPANA Injection should be administered cautiously and in reduced dosages to patients with creatinine clearance rate less than 50 mL/min.
- Use with CNS depressants
- OPANA Injection, like all opioid analgesics, should be started at 1/3 to 1/2 of the usual dose in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol because respiratory depression, hypotension and profound sedation or coma may result. No specific interaction between oxymorphone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate (see PRECAUTIONS: GENERAL andPRECAUTIONS: DRUG-DRUG INTERACTIONS)
- Geriatrics
- Caution should be exercised in the selection of the starting dose of OPANA Injection for an elderly patient starting at the low end of the dosing range.
- Maintenance of Therapy
- OPANA Injection is intended as an opioid analgesic for the management of moderate to severe pain where the use of an opioid analgesic is appropriate. During therapy, continual re-evaluation of the patient receiving OPANA Injection is important, with special attention to the maintenance of pain control and the relative incidence of side effects associated with therapy. If the level of pain increases, effort should be made to identify the source of increased pain, while adjusting the dose and/or using adjuvant analgesics such as acetaminophen or NSAIDs.
- Cessation of Therapy
- When the patient no longer requires therapy with OPANA Injection, doses should be tapered gradually to prevent signs and symptoms of withdrawal in the physically dependent patient.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oxymorphone (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Oxymorphone (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Oxymorphone (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oxymorphone (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Oxymorphone (injection) in pediatric patients.
Contraindications
- Condition1
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Oxymorphone (injection) in the drug label.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Oxymorphone (injection) in the drug label.
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Oxymorphone (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Oxymorphone (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Oxymorphone (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Oxymorphone (injection) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Oxymorphone (injection) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Oxymorphone (injection) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Oxymorphone (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Oxymorphone (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Oxymorphone (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Oxymorphone (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Oxymorphone (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Oxymorphone (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Oxymorphone (injection) in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Oxymorphone (injection) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Oxymorphone (injection) in the drug label.
Pharmacology
There is limited information regarding Oxymorphone (injection) Pharmacology in the drug label.
Mechanism of Action
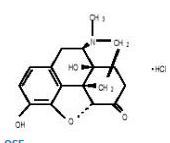
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Oxymorphone (injection) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Oxymorphone (injection) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Oxymorphone (injection) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Oxymorphone (injection) in the drug label.
How Supplied
Storage
There is limited information regarding Oxymorphone (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Oxymorphone (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Oxymorphone (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Oxymorphone (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Oxymorphone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Oxymorphone (injection) |Label Name=Oxymorphone (injection)11.png
}}
{{#subobject:
|Label Page=Oxymorphone (injection) |Label Name=Oxymorphone (injection)11.png
}}