Tranexamic acid (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 22: | Line 22: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | ||
|contraindications=* In patients with acquired defective color vision, since this prohibits measuring one endpoint that should be followed as a measure of toxicity. | |contraindications=CYKLOKAPRON Injection is contraindicated: | ||
* In patients with acquired defective color vision, since this prohibits measuring one endpoint that should be followed as a measure of toxicity. | |||
* In patients with [[subarachnoid hemorrhage]]. Anecdotal experience indicates that [[cerebral edema]] and [[cerebral infarction]] may be caused by CYKLOKAPRON in such patients. | * In patients with [[subarachnoid hemorrhage]]. Anecdotal experience indicates that [[cerebral edema]] and [[cerebral infarction]] may be caused by CYKLOKAPRON in such patients. | ||
* In patients with active intravascular clotting. | * In patients with active intravascular clotting. | ||
Revision as of 16:11, 27 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tranexamic acid (injection) is an antifibrinolytic agent that is FDA approved for the treatment of patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction. Common adverse reactions include abdominal pain, anemia, arthralgia, backache, cramps, musculoskeletal pain, headache, migraine, nasal sinus problem and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- CYKLOKAPRON Injection is indicated in patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction.
Dosage
- Immediately before tooth extraction in patients with hemophilia, administer 10 mg per kg body weight of CYKLOKAPRON intravenously together with replacement therapy.
- Following tooth extraction, intravenous therapy, at a dose of 10 mg per kg body weight three to four times daily, may be used for 2 to 8 days.
- Note: For patients with moderate to severe impaired renal function, the following dosages are recommended:
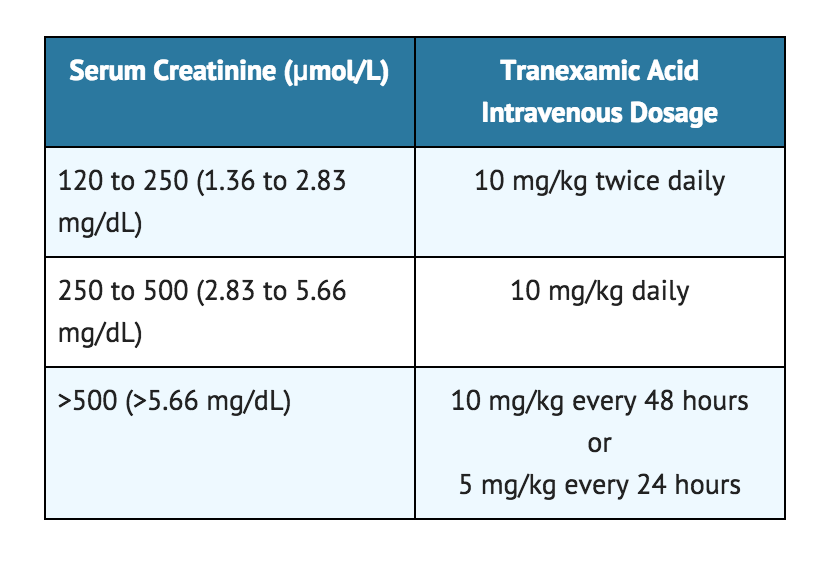
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tranexamic acid in adult patients.
Non–Guideline-Supported Use
- Dental surgical procedure - Hemophilia - Hemorrhage. [1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing CYKLOKAPRON therapy.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tranexamic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tranexamic acid in pediatric patients.
Contraindications
CYKLOKAPRON Injection is contraindicated:
- In patients with acquired defective color vision, since this prohibits measuring one endpoint that should be followed as a measure of toxicity.
- In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by CYKLOKAPRON in such patients.
- In patients with active intravascular clotting.
- In patients with hypersensitivity to tranexamic acid or any of the ingredients.
Warnings
There is limited information regarding Tranexamic acid (injection) Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Tranexamic acid (injection) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Tranexamic acid (injection) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Tranexamic acid (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Tranexamic acid (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tranexamic acid (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tranexamic acid (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Tranexamic acid (injection) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Tranexamic acid (injection) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Tranexamic acid (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Tranexamic acid (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tranexamic acid (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tranexamic acid (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tranexamic acid (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tranexamic acid (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tranexamic acid (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tranexamic acid (injection) Administration in the drug label.
Monitoring
There is limited information regarding Tranexamic acid (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tranexamic acid (injection) and IV administrations.
Overdosage
There is limited information regarding Tranexamic acid (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Tranexamic acid (injection) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Tranexamic acid (injection) Mechanism of Action in the drug label.
Structure
There is limited information regarding Tranexamic acid (injection) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Tranexamic acid (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Tranexamic acid (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Tranexamic acid (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Tranexamic acid (injection) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Tranexamic acid (injection) How Supplied in the drug label.
Storage
There is limited information regarding Tranexamic acid (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tranexamic acid (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tranexamic acid (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Tranexamic acid (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Tranexamic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Tranexamic acid (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Tranexamic acid (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Sindet-Pedersen S, Stenbjerg S, Ingerslev J (1988). "Control of gingival hemorrhage in hemophilic patients by inhibition of fibrinolysis with tranexamic acid". J Periodontal Res. 23 (1): 72–4. PMID 2963908.