Metirosine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag= | ||
{{VP}} | |||
<!-- | <!--Overview--> | ||
|genericName= | |||
| | |||
<!-- | |||
| | |||
|aOrAn= | |||
| | |||
| | a | ||
| | |||
| | |drugClass= | ||
| | |||
| | |||
| | |||
|indication= | |||
pheochromocytoma | |||
|hasBlackBoxWarning= | |||
Yes | |||
|adverseReactions= | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Pheochromocytoma===== | |||
*DEMSER is indicated in the treatment of patients with pheochromocytoma for: | |||
:*Preoperative preparation of patients for surgery | |||
:*Management of patients when surgery is contraindicated | |||
:*Chronic treatment of patients with malignant pheochromocytoma. | |||
*DEMSER is not recommended for the control of essential hypertension. | |||
*The recommended initial dosage of DEMSER for adults and children 12 years of age and older is 250 mg orally four times daily. This may be increased by 250 mg to 500 mg every day to a maximum of 4.0 g/day in divided doses. When used for preoperative preparation, the optimally effective dosage of DEMSER should be given for at least five to seven days. | |||
*Optimally effective dosages of DEMSER usually are between 2.0 and 3.0 g/day, and the dose should be titrated by monitoring clinical symptoms and catecholamine excretion. In patients who are hypertensive, dosage should be titrated to achieve normalization of blood pressure and control of clinical symptoms. In patients who are usually normotensive, dosage should be titrated to the amount that will reduce urinary metanephrines and/or vanillylmandelic acid by 50 percent or more. | |||
*If patients are not adequately controlled by the use of DEMSER, an alpha-adrenergic blocking agent (phenoxybenzamine) should be added. | |||
*Use of DEMSER in children under 12 years of age has been limited and a dosage schedule for this age group cannot be given. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
* DEMSER is contraindicated in persons known to be hypersensitive to this compound. | |||
<!--Warnings--> | |||
|warnings= | |||
* Maintain Fluid Volume During and After Surgery | |||
:*When DEMSER is used preoperatively, alone or especially in combination with alpha-adrenergic blocking drugs, adequate intravascular volume must be maintained intraoperatively (especially after tumor removal) and postoperatively to avoid hypotension and decreased perfusion of vital organs resulting from vasodilatation and expanded volume capacity. Following tumor removal, large volumes of plasma may be needed to maintain blood pressure and central venous pressure within the normal range. | |||
:*In addition, life-threatening arrhythmias may occur during anesthesia and surgery, and may require treatment with a beta-blocker or lidocaine. During surgery, patients should have continuous monitoring of blood pressure and electrocardiogram. | |||
*Intraoperative Effects | |||
:*While the preoperative use of DEMSER in patients with pheochromocytoma is thought to decrease intraoperative problems with blood pressure control, DEMSER does not eliminate the danger of hypertensive crises or arrhythmias during manipulation of the tumor, and the alpha-adrenergic blocking drug, phentolamine, may be needed. | |||
*Interaction with Alcohol | |||
:*DEMSER may add to the sedative effects of alcohol and other CNS depressants, e.g., hypnotics, sedatives, and tranquilizers. | |||
====Precautions==== | |||
* Metyrosine Crystalluria | |||
:*Crystalluria and urolithiasis have been found in dogs treated with DEMSER (Metyrosine) at doses similar to those used in humans, and crystalluria has also been observed in a few patients. To minimize the risk of crystalluria, patients should be urged to maintain water intake sufficient to achieve a daily urine volume of 2000 mL or more, particularly when doses greater than 2 g per day are given. Routine examination of the urine should be carried out. Metyrosine will crystallize as needles or rods. If metyrosine crystalluria occurs, fluid intake should be increased further. If crystalluria persists, the dosage should be reduced or the drug discontinued. | |||
*Relatively Little Data Regarding Long-term Use | |||
:*The total human experience with the drug is quite limited and few patients have been studied long-term. Chronic animal studies have not been carried out. Therefore, suitable laboratory tests should be carried out periodically in patients requiring prolonged use of DEMSER and caution should be observed in patients with impaired hepatic or renal function. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*Central Nervous System | |||
*Sedation: | |||
:*The most common adverse reaction to DEMSER is moderate to severe sedation, which has been observed in almost all patients. It occurs at both low and high dosages. Sedative effects begin within the first 24 hours of therapy, are maximal after two to three days, and tend to wane during the next few days. Sedation usually is not obvious after one week unless the dosage is increased, but at dosages greater than 2000 mg/day some degree of sedation or fatigue may persist. | |||
:*In most patients who experience sedation, temporary changes in sleep pattern occur following withdrawal of the drug. Changes consist of insomnia that may last for two or three days and feelings of increased alertness and ambition. Even patients who do not experience sedation while on DEMSER may report symptoms of psychic stimulation when the drug is discontinued. | |||
*Extrapyramidal Signs: | |||
:*Extrapyramidal signs such as drooling, speech difficulty, and tremor have been reported in approximately 10 percent of patients. These occasionally have been accompanied by trismus and frank parkinsonism. | |||
*Anxiety and Psychic Disturbances: | |||
:*Anxiety and psychic disturbances such as depression, hallucinations, disorientation, and confusion may occur. These effects seem to be dose-dependent and may disappear with reduction of dosage. | |||
*Diarrhea | |||
:*Diarrhea occurs in about 10 percent of patients and may be severe. Anti-diarrheal agents may be required if continuation of DEMSER is necessary. | |||
*Miscellaneous | |||
:*Infrequently, slight swelling of the breast, galactorrhea, nasal stuffiness, decreased salivation, dry mouth, headache, nausea, vomiting, abdominal pain, and impotence or failure of ejaculation may occur. Crystalluria (see PRECAUTIONS) and transient dysuria and hematuria have been observed in a few patients. Hematologic disorders (including eosinophilia, anemia, thrombocytopenia, and thrombocytosis), increased SGOT levels, peripheral edema, and hypersensitivity reactions such as urticaria and pharyngeal edema have been reported rarely. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
*Caution should be observed in administering DEMSER to patients receiving [[phenothiazines]] or [[haloperidol]] because the extrapyramidal effects of these drugs can be expected to be potentiated by inhibition of catecholamine synthesis. | |||
*Concurrent use of DEMSER with alcohol or other CNS depressants can increase their sedative effects. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category C''' | |||
*Animal reproduction studies have not been conducted with DEMSER. It is also not known whether DEMSER can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. DEMSER should be given to a pregnant woman only if clearly needed. | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
*It is not known whether DEMSER is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DEMSER is administered to a nursing woman. | |||
|useInPed= | |||
*Safety and effectiveness in pediatric patients below the age of 12 years have not been established. | |||
|useInGeri= | |||
*Clinical studies of DEMSER did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
*Signs of metyrosine overdosage include those central nervous system effects observed in some patients even at low dosages. | |||
*At doses exceeding 2000 mg/day, some degree of sedation or feeling of fatigue may persist. Doses of 2000-4000 mg/day can result in anxiety or agitated depression, neuromuscular effects (including fine tremor of the hands, gross tremor of the trunk, tightening of the jaw with trismus), diarrhea, and decreased salivation with dry mouth. | |||
*Reduction of drug dose or cessation of treatment results in the disappearance of these symptoms. | |||
*The acute toxicity of metyrosine was 442 mg/kg and 752 mg/kg in the female mouse and rat respectively. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* DEMSER inhibits tyrosine hydroxylase, which catalyzes the first transformation in catecholamine biosynthesis, i.e., the conversion of tyrosine to dihydroxyphenylalanine (DOPA). Because the first step is also the rate-limiting step, blockade of tyrosine hydroxylase activity results in decreased endogenous levels of catecholamines, usually measured as decreased urinary excretion of catecholamines and their metabolites. | |||
<!--Structure--> | |||
|structure= | |||
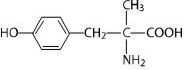
* DEMSER1 (Metyrosine) is (–)-α-methyl-L-tyrosine or (α-MPT). It has the following structural formula: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Metyrosine is a white, crystalline compound of molecular weight 195. It is very slightly soluble in water, acetone, and methanol, and insoluble in chloroform and benzene. It is soluble in acidic aqueous solutions. It is also soluble in alkaline aqueous solutions, but is subject to oxidative degradation under these conditions. | |||
*DEMSER is supplied as capsules, for oral administration. Each capsule contains 250 mg metyrosine. Inactive ingredients are colloidal silicon dioxide, gelatin, hydroxypropyl cellulose, magnesium stearate, titanium dioxide, and FD&C Blue 2. | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
*In patients with pheochromocytoma, who produce excessive amounts of norepinephrine and epinephrine, administration of one to four grams of DEMSER per day has reduced catecholamine biosynthesis from about 35 to 80 percent as measured by the total excretion of catecholamines and their metabolites (metanephrine and vanillylmandelic acid). The maximum biochemical effect usually occurs within two to three days, and the urinary concentration of catecholamines and their metabolites usually returns to pretreatment levels within three to four days after DEMSER is discontinued. In some patients the total excretion of catecholamines and catecholamine metabolites may be lowered to normal or near normal levels (less than 10 mg/24 hours). In most patients the duration of treatment has been two to eight weeks, but several patients have received DEMSER for periods of one to 10 years. Most patients with pheochromocytoma treated with DEMSER experience decreased frequency and severity of hypertensive attacks with their associated headache, nausea, sweating, and tachycardia. In patients who respond, blood pressure decreases progressively during the first two days of therapy with DEMSER; after withdrawal, blood pressure usually increases gradually to pretreatment values within two to three days. | |||
*Metyrosine is well absorbed from the gastrointestinal tract. From 53 to 88 percent (mean 69 percent) was recovered in the urine as unchanged drug following maintenance oral doses of 600 to 4000 mg/24 hours in patients with pheochromocytoma or essential hypertension. Less than 1% of the dose was recovered as catechol metabolites. These metabolites are probably not present in sufficient amounts to contribute to the biochemical effects of metyrosine. The quantities excreted, however, are sufficient to interfere with accurate determination of urinary catecholamines determined by routine techniques. | |||
*Plasma half-life of metyrosine determined over an 8-hour period after single oral doses was 3-3.7 hours in three patients. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
*Long-term carcinogenic studies in animals and studies on mutagenesis and impairment of fertility have not been performed with metyrosine. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
*When receiving DEMSER, patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a motor vehicle or operating machinery. DEMSER may have additive sedative effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, and tranquilizers. | |||
*Patients should be advised to maintain a liberal fluid intake. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | }} | ||
<!--Pill Image--> | |||
= | {{PillImage | ||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
= | {{LabelImage | ||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
= | {{LabelImage | ||
{{ | |fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | ||
}} | |||
<!--Category--> | |||
[[Category:Drug]] | [[Category:Drug]] | ||
Revision as of 16:37, 16 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Metirosine is a that is FDA approved for the {{{indicationType}}} of pheochromocytoma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Pheochromocytoma
- DEMSER is indicated in the treatment of patients with pheochromocytoma for:
- Preoperative preparation of patients for surgery
- Management of patients when surgery is contraindicated
- Chronic treatment of patients with malignant pheochromocytoma.
- DEMSER is not recommended for the control of essential hypertension.
- The recommended initial dosage of DEMSER for adults and children 12 years of age and older is 250 mg orally four times daily. This may be increased by 250 mg to 500 mg every day to a maximum of 4.0 g/day in divided doses. When used for preoperative preparation, the optimally effective dosage of DEMSER should be given for at least five to seven days.
- Optimally effective dosages of DEMSER usually are between 2.0 and 3.0 g/day, and the dose should be titrated by monitoring clinical symptoms and catecholamine excretion. In patients who are hypertensive, dosage should be titrated to achieve normalization of blood pressure and control of clinical symptoms. In patients who are usually normotensive, dosage should be titrated to the amount that will reduce urinary metanephrines and/or vanillylmandelic acid by 50 percent or more.
- If patients are not adequately controlled by the use of DEMSER, an alpha-adrenergic blocking agent (phenoxybenzamine) should be added.
- Use of DEMSER in children under 12 years of age has been limited and a dosage schedule for this age group cannot be given.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Metirosine in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Metirosine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Metirosine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Metirosine in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Metirosine in pediatric patients.
Contraindications
- DEMSER is contraindicated in persons known to be hypersensitive to this compound.
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Maintain Fluid Volume During and After Surgery
- When DEMSER is used preoperatively, alone or especially in combination with alpha-adrenergic blocking drugs, adequate intravascular volume must be maintained intraoperatively (especially after tumor removal) and postoperatively to avoid hypotension and decreased perfusion of vital organs resulting from vasodilatation and expanded volume capacity. Following tumor removal, large volumes of plasma may be needed to maintain blood pressure and central venous pressure within the normal range.
- In addition, life-threatening arrhythmias may occur during anesthesia and surgery, and may require treatment with a beta-blocker or lidocaine. During surgery, patients should have continuous monitoring of blood pressure and electrocardiogram.
- Intraoperative Effects
- While the preoperative use of DEMSER in patients with pheochromocytoma is thought to decrease intraoperative problems with blood pressure control, DEMSER does not eliminate the danger of hypertensive crises or arrhythmias during manipulation of the tumor, and the alpha-adrenergic blocking drug, phentolamine, may be needed.
- Interaction with Alcohol
- DEMSER may add to the sedative effects of alcohol and other CNS depressants, e.g., hypnotics, sedatives, and tranquilizers.
Precautions
- Metyrosine Crystalluria
- Crystalluria and urolithiasis have been found in dogs treated with DEMSER (Metyrosine) at doses similar to those used in humans, and crystalluria has also been observed in a few patients. To minimize the risk of crystalluria, patients should be urged to maintain water intake sufficient to achieve a daily urine volume of 2000 mL or more, particularly when doses greater than 2 g per day are given. Routine examination of the urine should be carried out. Metyrosine will crystallize as needles or rods. If metyrosine crystalluria occurs, fluid intake should be increased further. If crystalluria persists, the dosage should be reduced or the drug discontinued.
- Relatively Little Data Regarding Long-term Use
- The total human experience with the drug is quite limited and few patients have been studied long-term. Chronic animal studies have not been carried out. Therefore, suitable laboratory tests should be carried out periodically in patients requiring prolonged use of DEMSER and caution should be observed in patients with impaired hepatic or renal function.
Adverse Reactions
Clinical Trials Experience
- Central Nervous System
- Sedation:
- The most common adverse reaction to DEMSER is moderate to severe sedation, which has been observed in almost all patients. It occurs at both low and high dosages. Sedative effects begin within the first 24 hours of therapy, are maximal after two to three days, and tend to wane during the next few days. Sedation usually is not obvious after one week unless the dosage is increased, but at dosages greater than 2000 mg/day some degree of sedation or fatigue may persist.
- In most patients who experience sedation, temporary changes in sleep pattern occur following withdrawal of the drug. Changes consist of insomnia that may last for two or three days and feelings of increased alertness and ambition. Even patients who do not experience sedation while on DEMSER may report symptoms of psychic stimulation when the drug is discontinued.
- Extrapyramidal Signs:
- Extrapyramidal signs such as drooling, speech difficulty, and tremor have been reported in approximately 10 percent of patients. These occasionally have been accompanied by trismus and frank parkinsonism.
- Anxiety and Psychic Disturbances:
- Anxiety and psychic disturbances such as depression, hallucinations, disorientation, and confusion may occur. These effects seem to be dose-dependent and may disappear with reduction of dosage.
- Diarrhea
- Diarrhea occurs in about 10 percent of patients and may be severe. Anti-diarrheal agents may be required if continuation of DEMSER is necessary.
- Miscellaneous
- Infrequently, slight swelling of the breast, galactorrhea, nasal stuffiness, decreased salivation, dry mouth, headache, nausea, vomiting, abdominal pain, and impotence or failure of ejaculation may occur. Crystalluria (see PRECAUTIONS) and transient dysuria and hematuria have been observed in a few patients. Hematologic disorders (including eosinophilia, anemia, thrombocytopenia, and thrombocytosis), increased SGOT levels, peripheral edema, and hypersensitivity reactions such as urticaria and pharyngeal edema have been reported rarely.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Metirosine in the drug label.
Drug Interactions
- Caution should be observed in administering DEMSER to patients receiving phenothiazines or haloperidol because the extrapyramidal effects of these drugs can be expected to be potentiated by inhibition of catecholamine synthesis.
- Concurrent use of DEMSER with alcohol or other CNS depressants can increase their sedative effects.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Animal reproduction studies have not been conducted with DEMSER. It is also not known whether DEMSER can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. DEMSER should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Metirosine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Metirosine during labor and delivery.
Nursing Mothers
- It is not known whether DEMSER is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DEMSER is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
Geriatic Use
- Clinical studies of DEMSER did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Metirosine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Metirosine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Metirosine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Metirosine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Metirosine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Metirosine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Metirosine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Metirosine in the drug label.
Overdosage
Acute Overdose
- Signs of metyrosine overdosage include those central nervous system effects observed in some patients even at low dosages.
- At doses exceeding 2000 mg/day, some degree of sedation or feeling of fatigue may persist. Doses of 2000-4000 mg/day can result in anxiety or agitated depression, neuromuscular effects (including fine tremor of the hands, gross tremor of the trunk, tightening of the jaw with trismus), diarrhea, and decreased salivation with dry mouth.
- Reduction of drug dose or cessation of treatment results in the disappearance of these symptoms.
- The acute toxicity of metyrosine was 442 mg/kg and 752 mg/kg in the female mouse and rat respectively.
Chronic Overdose
There is limited information regarding Chronic Overdose of Metirosine in the drug label.
Pharmacology
There is limited information regarding Metirosine Pharmacology in the drug label.
Mechanism of Action
- DEMSER inhibits tyrosine hydroxylase, which catalyzes the first transformation in catecholamine biosynthesis, i.e., the conversion of tyrosine to dihydroxyphenylalanine (DOPA). Because the first step is also the rate-limiting step, blockade of tyrosine hydroxylase activity results in decreased endogenous levels of catecholamines, usually measured as decreased urinary excretion of catecholamines and their metabolites.
Structure
- DEMSER1 (Metyrosine) is (–)-α-methyl-L-tyrosine or (α-MPT). It has the following structural formula:
- Metyrosine is a white, crystalline compound of molecular weight 195. It is very slightly soluble in water, acetone, and methanol, and insoluble in chloroform and benzene. It is soluble in acidic aqueous solutions. It is also soluble in alkaline aqueous solutions, but is subject to oxidative degradation under these conditions.
- DEMSER is supplied as capsules, for oral administration. Each capsule contains 250 mg metyrosine. Inactive ingredients are colloidal silicon dioxide, gelatin, hydroxypropyl cellulose, magnesium stearate, titanium dioxide, and FD&C Blue 2.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Metirosine in the drug label.
Pharmacokinetics
- In patients with pheochromocytoma, who produce excessive amounts of norepinephrine and epinephrine, administration of one to four grams of DEMSER per day has reduced catecholamine biosynthesis from about 35 to 80 percent as measured by the total excretion of catecholamines and their metabolites (metanephrine and vanillylmandelic acid). The maximum biochemical effect usually occurs within two to three days, and the urinary concentration of catecholamines and their metabolites usually returns to pretreatment levels within three to four days after DEMSER is discontinued. In some patients the total excretion of catecholamines and catecholamine metabolites may be lowered to normal or near normal levels (less than 10 mg/24 hours). In most patients the duration of treatment has been two to eight weeks, but several patients have received DEMSER for periods of one to 10 years. Most patients with pheochromocytoma treated with DEMSER experience decreased frequency and severity of hypertensive attacks with their associated headache, nausea, sweating, and tachycardia. In patients who respond, blood pressure decreases progressively during the first two days of therapy with DEMSER; after withdrawal, blood pressure usually increases gradually to pretreatment values within two to three days.
- Metyrosine is well absorbed from the gastrointestinal tract. From 53 to 88 percent (mean 69 percent) was recovered in the urine as unchanged drug following maintenance oral doses of 600 to 4000 mg/24 hours in patients with pheochromocytoma or essential hypertension. Less than 1% of the dose was recovered as catechol metabolites. These metabolites are probably not present in sufficient amounts to contribute to the biochemical effects of metyrosine. The quantities excreted, however, are sufficient to interfere with accurate determination of urinary catecholamines determined by routine techniques.
- Plasma half-life of metyrosine determined over an 8-hour period after single oral doses was 3-3.7 hours in three patients.
Nonclinical Toxicology
- Long-term carcinogenic studies in animals and studies on mutagenesis and impairment of fertility have not been performed with metyrosine.
Clinical Studies
There is limited information regarding Clinical Studies of Metirosine in the drug label.
How Supplied
Storage
There is limited information regarding Metirosine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Metirosine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Metirosine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- When receiving DEMSER, patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a motor vehicle or operating machinery. DEMSER may have additive sedative effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, and tranquilizers.
- Patients should be advised to maintain a liberal fluid intake.
Precautions with Alcohol
- Alcohol-Metirosine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Metirosine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Metirosine |Label Name=Metirosine11.png
}}
{{#subobject:
|Label Page=Metirosine |Label Name=Metirosine11.png
}}