Tetracaine (ophthalmic): Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{AP}} | |||
|genericName=Tetracaine | |||
|aOrAn=a | |||
|drugClass=[[local anesthesic]] | |||
|indication=[[tonometry]], [[gonioscopy]], removal of corneal foreign bodies, [[conjunctival scraping]] for diagnostic purposes, suture removal from the [[cornea]] or [[conjunctiva]], other short corneal and conjunctival procedures | |||
|adverseReactions=[[nausea]], [[vomiting]] and burning sensation in eye | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult======For Tonometry and Other Procedures of Short Duration===== | |||
Instill one or two drops just prior to evaluation. | |||
=====For Minor Surgical Procedures such as Foreign Body or Suture Removal===== | |||
Instill one or two drops every five to ten minutes for one to three doses. | |||
=====For Prolonged Anesthesia as in Cataract Extraction===== | |||
Instill one or two drops in the eye(s) every five to ten minutes for three to five doses. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tetracaine in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Tetracaine in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tetracaine in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Tetracaine in pediatric patients. | |||
|contraindications=Should not be used by the patient without physician supervision, or in those persons showing [[hypersensitivity]] to any component of this preparation. | |||
|warnings=For topical ophthalmic use only. Not for parenteral use. Not for injection. Do not use solution if it contains crystals or if it is cloudy or discolored. Prolonged use results in diminished duration of anesthesia and retarded healing. This may cause the drug to be used more frequently, creating a “vicious circle”. Subsequent corneal infection and/or corneal opacification with accompanying permanent visual loss or [[corneal perforation]] may occur. Prolonged use may also produce severe [[keratitis]]. | |||
|clinicalTrials=Transient symptoms (signs) such as [[stinging]], burning and [[conjunctival redness]] may occur. A rare, severe, immediate type [[allergic corneal reaction]] has been reported characterized by acute diffuse [[epithelial keratitis]] with filament formation and/or sloughing of large areas of [[necrotic epithelium]], diffuse stromal edema, [[descemetitis]] and [[iritis]]. | |||
TO REPORT SUSPECTED ADVERSE REACTIONS, contact Altaire Pharmaceuticals, Inc., at 1-800-258-2471 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Animal reproduction studies have not been performed with Tetracaine Hydrochloride. It is also not known whether Tetracaine Hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% should be given to pregnant women only if clearly needed. | |||
|useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is administered to a nursing mother. | |||
|useInPed=Safety and effectiveness in children have not been established. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 470603479 | |||
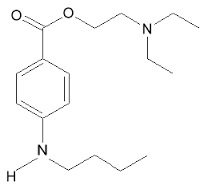
| IUPAC_name = 2-(dimethylamino)ethyl 4-(butylamino)benzoate | |||
| image = Tetracaine Structure.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|monograph|tetracaine}} | |||
| MedlinePlus = a682640 | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = Rx Only | |||
| routes_of_administration = Topical, Epidural, Spinal | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 75.6 | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 94-24-6 | |||
| CAS_supplemental = <br/>{{CAS|136-47-0}} ([[hydrochloride]]) <!-- Also CAS verified --> | |||
| ATC_prefix = C05 | |||
| ATC_suffix = AD02 | |||
| ATC_supplemental = {{ATC|D04|AB06}} {{ATC|N01|BA03}} {{ATC|S01|HA03}} | |||
| PubChem = 5411 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 5218 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 0619F35CGV | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00551 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 9468 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 698 | |||
<!--Chemical data--> | |||
| C=15 | H=24 | N=2 | O=2 | |||
| molecular_weight = 264.363 g/mol | |||
| smiles = O=C(OCCN(C)C)c1ccc(NCCCC)cc1 | |||
| InChI = 1/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3 | |||
| InChIKey = GKCBAIGFKIBETG-UHFFFAOYAR | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = GKCBAIGFKIBETG-UHFFFAOYSA-N | |||
}} | |||
|structure=Chemical Name: | |||
Benzoic acid, 4-(butylamino)-,2-(dimethylamino)ethyl ester, monohydrochloride | |||
[[file:Tetracaine Structure.png|none|300px]] | |||
|PD=etracaine Hydrochloride Ophthalmic Solution, USP 0.5% acts by decreasing the permeability of the neuronal membrane, thereby decreasing the flux of sodium, potassium and other ions associated with propagation of the nerve impulse. The onset of anesthesia usually begins within 30 seconds and lasts a relatively short period of time. | |||
|howSupplied=Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is supplied in 15 mL and 30 mL single drop plastic containers. | |||
|storage=Store at room temperature, 15°-30°C (59°-86°F). Keep container tightly closed. Protect from light. | |||
|alcohol=Alcohol-Tetracaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
{{Drugbox | {{Drugbox | ||
| verifiedrevid = 470603479 | | verifiedrevid = 470603479 | ||
Revision as of 14:10, 26 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tetracaine (ophthalmic) is a local anesthesic that is FDA approved for the {{{indicationType}}} of tonometry, gonioscopy, removal of corneal foreign bodies, conjunctival scraping for diagnostic purposes, suture removal from the cornea or conjunctiva, other short corneal and conjunctival procedures. Common adverse reactions include nausea, vomiting and burning sensation in eye.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
For Tonometry and Other Procedures of Short Duration
Instill one or two drops just prior to evaluation.
For Minor Surgical Procedures such as Foreign Body or Suture Removal
Instill one or two drops every five to ten minutes for one to three doses.
For Prolonged Anesthesia as in Cataract Extraction
Instill one or two drops in the eye(s) every five to ten minutes for three to five doses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tetracaine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tetracaine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Tetracaine (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tetracaine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tetracaine in pediatric patients.
Contraindications
Should not be used by the patient without physician supervision, or in those persons showing hypersensitivity to any component of this preparation.
Warnings
For topical ophthalmic use only. Not for parenteral use. Not for injection. Do not use solution if it contains crystals or if it is cloudy or discolored. Prolonged use results in diminished duration of anesthesia and retarded healing. This may cause the drug to be used more frequently, creating a “vicious circle”. Subsequent corneal infection and/or corneal opacification with accompanying permanent visual loss or corneal perforation may occur. Prolonged use may also produce severe keratitis.
Adverse Reactions
Clinical Trials Experience
Transient symptoms (signs) such as stinging, burning and conjunctival redness may occur. A rare, severe, immediate type allergic corneal reaction has been reported characterized by acute diffuse epithelial keratitis with filament formation and/or sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
TO REPORT SUSPECTED ADVERSE REACTIONS, contact Altaire Pharmaceuticals, Inc., at 1-800-258-2471 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Postmarketing Experience
There is limited information regarding Tetracaine (ophthalmic) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Tetracaine (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been performed with Tetracaine Hydrochloride. It is also not known whether Tetracaine Hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% should be given to pregnant women only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tetracaine (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tetracaine (ophthalmic) during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is administered to a nursing mother.
Pediatric Use
Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Tetracaine (ophthalmic) in geriatric settings.
Gender
There is no FDA guidance on the use of Tetracaine (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tetracaine (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tetracaine (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tetracaine (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tetracaine (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tetracaine (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tetracaine (ophthalmic) Administration in the drug label.
Monitoring
There is limited information regarding Tetracaine (ophthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tetracaine (ophthalmic) and IV administrations.
Overdosage
There is limited information regarding Tetracaine (ophthalmic) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Tetracaine (ophthalmic)
| |
| Systematic (IUPAC) name | |
| 2-(dimethylamino)ethyl 4-(butylamino)benzoate | |
| Identifiers | |
| CAS number | 136-47-0 (hydrochloride) |
| ATC code | C05 D04AB06 (WHO) N01BA03 (WHO) S01HA03 (WHO) |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 264.363 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 75.6 |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Rx Only |
| Routes | Topical, Epidural, Spinal |
Mechanism of Action
There is limited information regarding Tetracaine (ophthalmic) Mechanism of Action in the drug label.
Structure
Chemical Name: Benzoic acid, 4-(butylamino)-,2-(dimethylamino)ethyl ester, monohydrochloride

Pharmacodynamics
etracaine Hydrochloride Ophthalmic Solution, USP 0.5% acts by decreasing the permeability of the neuronal membrane, thereby decreasing the flux of sodium, potassium and other ions associated with propagation of the nerve impulse. The onset of anesthesia usually begins within 30 seconds and lasts a relatively short period of time.
Pharmacokinetics
There is limited information regarding Tetracaine (ophthalmic) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Tetracaine (ophthalmic) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Tetracaine (ophthalmic) Clinical Studies in the drug label.
How Supplied
Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is supplied in 15 mL and 30 mL single drop plastic containers.
Storage
Store at room temperature, 15°-30°C (59°-86°F). Keep container tightly closed. Protect from light.
Images
Drug Images
{{#ask: Page Name::Tetracaine (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tetracaine (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Tetracaine (ophthalmic) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Tetracaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Tetracaine (ophthalmic) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Tetracaine (ophthalmic) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Tetracaine2DCSD.svg | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682640 |
| Routes of administration | Topical, Epidural, Spinal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75.6 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C15H24N2O2 |
| Molar mass | 264.363 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Tetracaine (INN, also known as amethocaine; trade name Pontocaine. Ametop and Dicaine) is a potent local anesthetic of the ester group. It is mainly used topically in ophthalmology and as an antipruritic, and it has been used in spinal anesthesia.
In biomedical research, tetracaine is used to alter the function of calcium release channels (ryanodine receptors) that control the release of calcium from intracellular stores. Tetracaine is an allosteric blocker of channel function. At low concentrations, tetracaine causes an initial inhibition of spontaneous calcium release events, while at high concentrations, tetracaine blocks release completely.[1]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[2]
Chemistry
Tetracaine is synthesized from 4-butylaminobenzoic acid. The ethyl ester is formed through an acid-catalyzed esterification reaction. Base-catalyzed transesterification is achieved by boiling the ethyl ester of 4-butylaminobenzoic acid with excess 2-dimethylaminoethanol in the presence of a small amount of sodium ethoxide.[3]

References
- ↑ Györke, S (1997). "Dual effects of tetracaine on spontaneous sodium release in rat ventricular myocytes". 500 (2). J Physiol: 297&ndash, 309. Unknown parameter
|coauthors=ignored (help) - ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Winthrop Chemical Company, Inc. U.S. Patent 1,889,645, 1932.
Further reading
- O. Eisleb, Template:US Patent (1932).
- Pages with script errors
- Pages with citations using unsupported parameters
- Pages with broken file links
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Local anesthetics
- Drug