Adrenocortical carcinoma pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
==Pathophysiology== | ==Pathophysiology== | ||
ACCs are typically large tumors upon clinical presentation, often measuring more than 6 cm in diameter (97). They are bilateral in 2% to 10% of cases (98, 99). Metastases to the liver, lungs, or lymph nodes can be seen, and invasion of adjacent organs or venous extension into the renal vein and/or inferior vena cava may be present. Inferior vena cava invasion has been reported in 9% to 19% of cases at presentation (98). Due to the presence of internal hemorrhage, necrosis, and calcifications, these tumors tend to vary in appearance with frequent heterogeneous enhancement. | |||
==Genetics== | |||
The genetic dissection of ACC has revealed genomic aberrations that contribute to neoplastic transformation of adrenocortical cells: | |||
'''''1. Clonality''''' | |||
* ACCs initiate from monoclonal cell populations, suggesting that mutation events lead to clonal expansion and ultimate progression to cancer (177, 178). | |||
* Flow cytometry revealed aneuploidy in ACC.(180).aneuploidy was observed in 75% of ACC. | |||
* Assessment of aneuploidy with histopathological criteria in 7 of 9 adrenal tumors revealed a high correlation with Weiss score >3 (indicative of malignancy) (182). | |||
* No significant difference in overall survival was observed in patients with ACC exhibiting aneuploidy vs patients with ACC exhibiting diploid neoplasms (126). | |||
'''''2. Gene expression arrays''''' | |||
Global gene expression studies aim to identify biomarkers that could provide diagnostic and prognostic utility in addition to the classic histological analyses and hold the promise of new potential targets for therapy. ACAs and ACCs have distinct expression profiles (174, 190– 192). An initial study identified elevated expression of genes involved in cell proliferation in ACCs, such as ''IGF2'', compared with increased expression of steroidogenic genes in ACAs (steroidogenic cluster) (190). Giordano et al (192) identified unique transcriptionally activated (12q and 5q) and repressed (11q, 1p, and 17p) chromosomal regions in 33 ACCs vs 22 ACAs in a microarray study, which confirmed the early chromosomal studies. More recently, 2 large studies have correlated expression profiles in ACC with clinical outcome. Specifically, Giordano et al (192) determined that ACCs with high histological grade exhibited marked overexpression of cell cycle and functional aneuploidy genes, which correlated with decreased overall survival. In another study, cluster analysis of ACCs again revealed 2 distinct groups with different genetic signatures and concomitant distinct clinical outcomes. ACCs with poor outcome were enriched for genes involved in cell cycle and proliferation, whereas ACCs in the better outcome group exhibited overexpression of genes involved in differentiation, metabolism, and intracellular transport. Expression levels of ''BUB1B'' and ''PINK1'' alone identified subgroups of ACCs with different overall survival, regardless of tumor stage. Similarly, the expression levels of ''DLG7'' and ''PINK1'' identified subgroups ofACCswith distinct disease-free survival, regardless of tumor grade (191). These findings were later validated in a separate cohort of adult patients (193). | |||
==Gross Pathology== | ==Gross Pathology== | ||
On gross pathology, adrenocortical carcinomas are often large, with a tan-yellow cut surface and areas of [[hemorrhage]] and [[necrosis]]. | On gross pathology, adrenocortical carcinomas are often large, with a tan-yellow cut surface and areas of [[hemorrhage]] and [[necrosis]]. | ||
Revision as of 18:07, 20 September 2017
|
Adrenocortical carcinoma Microchapters |
|
Differentiating Adrenocortical carcinoma from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Study |
|
Adrenocortical carcinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Adrenocortical carcinoma pathophysiology |
|
Risk calculators and risk factors for Adrenocortical carcinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2]Shivali Marketkar, M.B.B.S. [3]Ahmad Al Maradni, M.D. [4]
Overview
On gross pathology, a large tan-yellow surface with areas of hemorrhage and necrosis is a characteristic finding of adrenocortical carcinoma. On microscopic histopathological analysis, sheets of atypical cells with some resemblance to the cells of the normal adrenal cortex are a characteristic finding of adrenocortical carcinoma.
Pathophysiology
ACCs are typically large tumors upon clinical presentation, often measuring more than 6 cm in diameter (97). They are bilateral in 2% to 10% of cases (98, 99). Metastases to the liver, lungs, or lymph nodes can be seen, and invasion of adjacent organs or venous extension into the renal vein and/or inferior vena cava may be present. Inferior vena cava invasion has been reported in 9% to 19% of cases at presentation (98). Due to the presence of internal hemorrhage, necrosis, and calcifications, these tumors tend to vary in appearance with frequent heterogeneous enhancement.
Genetics
The genetic dissection of ACC has revealed genomic aberrations that contribute to neoplastic transformation of adrenocortical cells:
1. Clonality
- ACCs initiate from monoclonal cell populations, suggesting that mutation events lead to clonal expansion and ultimate progression to cancer (177, 178).
- Flow cytometry revealed aneuploidy in ACC.(180).aneuploidy was observed in 75% of ACC.
- Assessment of aneuploidy with histopathological criteria in 7 of 9 adrenal tumors revealed a high correlation with Weiss score >3 (indicative of malignancy) (182).
- No significant difference in overall survival was observed in patients with ACC exhibiting aneuploidy vs patients with ACC exhibiting diploid neoplasms (126).
2. Gene expression arrays
Global gene expression studies aim to identify biomarkers that could provide diagnostic and prognostic utility in addition to the classic histological analyses and hold the promise of new potential targets for therapy. ACAs and ACCs have distinct expression profiles (174, 190– 192). An initial study identified elevated expression of genes involved in cell proliferation in ACCs, such as IGF2, compared with increased expression of steroidogenic genes in ACAs (steroidogenic cluster) (190). Giordano et al (192) identified unique transcriptionally activated (12q and 5q) and repressed (11q, 1p, and 17p) chromosomal regions in 33 ACCs vs 22 ACAs in a microarray study, which confirmed the early chromosomal studies. More recently, 2 large studies have correlated expression profiles in ACC with clinical outcome. Specifically, Giordano et al (192) determined that ACCs with high histological grade exhibited marked overexpression of cell cycle and functional aneuploidy genes, which correlated with decreased overall survival. In another study, cluster analysis of ACCs again revealed 2 distinct groups with different genetic signatures and concomitant distinct clinical outcomes. ACCs with poor outcome were enriched for genes involved in cell cycle and proliferation, whereas ACCs in the better outcome group exhibited overexpression of genes involved in differentiation, metabolism, and intracellular transport. Expression levels of BUB1B and PINK1 alone identified subgroups of ACCs with different overall survival, regardless of tumor stage. Similarly, the expression levels of DLG7 and PINK1 identified subgroups ofACCswith distinct disease-free survival, regardless of tumor grade (191). These findings were later validated in a separate cohort of adult patients (193).
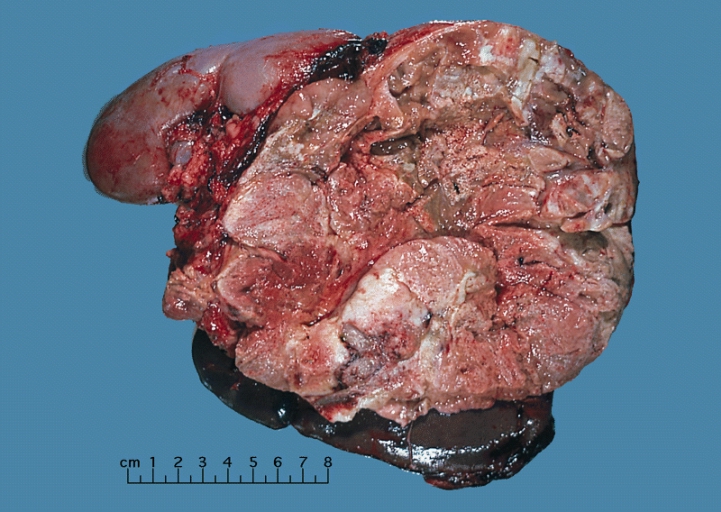
Gross Pathology
On gross pathology, adrenocortical carcinomas are often large, with a tan-yellow cut surface and areas of hemorrhage and necrosis.
Shown above is a large adrenal cortical carcinoma resected from a 27-year-old woman. The tumor measured 17 cm in diameter and invaded kidney and spleen which necessitated en bloc removal of these organs with tumor. Patient had evidence of virilization.
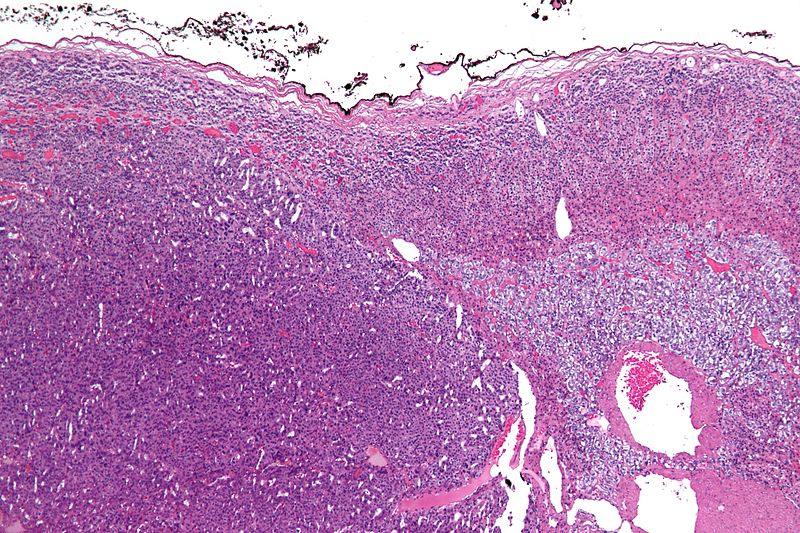
Microscopic Pathology
On microscopic examination, the tumor usually displays sheets of atypical cells with some resemblance to the cells of the normal adrenal cortex. The presence of invasion and mitotic activity helps differentiate small cancers from adrenocortical adenomas.[1]
The Weiss criteria of adrenocortical malignancy comprise the most reliable histopathological scoring system differentiating ACC from ACA 9–11
ACC can be diagnosed by the presence of at least 3 of the 9 Weiss criteria:
- Three relate to cytological features (nuclear grade, mitoses and atypical mitoses)
- Three refer to tumor structure (clear cells, diffuse architecture, and confluent necrosis)
- Three relate to invasion (venous invasion, sinusoidal invasion, and capsular infiltration)
Micrograph of an adrenocortical carcinoma (left of image - dark blue) and the adrenal cortex it arose from (right-top of image - pink/light blue). Benign adrenal medulla is present (right-middle of image - gray/blue). H&E stain.
Video
Shown below is a video explaining the histology of adrenocortical carcinoma
{{#ev:youtube|7jMFENhPaOM}}
References
- ↑ Richard Cote, Saul Suster, Lawrence Weiss, Noel Weidner (Editor). Modern Surgical Pathology (2 Volume Set). London: W B Saunders. ISBN 0-7216-7253-1.