Bosentan: Difference between revisions
No edit summary |
No edit summary |
||
| Line 186: | Line 186: | ||
* Treatment discontinuations due to adverse events other than those related to pulmonary hypertension during the clinical trials in patients with pulmonary arterial hypertension were more frequent on Tracleer (6%; 15/258 patients) than on placebo (3%; 5/172 patients). In this database the only cause of discontinuations > 1% and occurring more often on Tracleer was abnormal liver function. | * Treatment discontinuations due to adverse events other than those related to pulmonary hypertension during the clinical trials in patients with pulmonary arterial hypertension were more frequent on Tracleer (6%; 15/258 patients) than on placebo (3%; 5/172 patients). In this database the only cause of discontinuations > 1% and occurring more often on Tracleer was abnormal liver function. | ||
* The adverse drug events that occurred in ≥3% of the Tracleer-treated patients and were more common on Tracleer in placebo-controlled trials in pulmonary arterial hypertension at doses of 125 or 250 mg twice daily are shown in Table 2: | * The adverse drug events that occurred in ≥3% of the Tracleer-treated patients and were more common on Tracleer in placebo-controlled trials in pulmonary arterial hypertension at doses of 125 or 250 mg twice daily are shown in Table 2: | ||
: [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
=====Decreased Sperm Counts===== | =====Decreased Sperm Counts===== | ||
Revision as of 01:01, 27 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISKS OF HEPATOTOXICITY and TERATOGENICITY
See full prescribing information for complete Boxed Warning.
* Because of the risks of hepatotoxicity and birth defects, Tracleer is available only through a restricted program called the Tracleer Access Program (T.A.P.). T.A.P. is a component of the Tracleer Risk Evaluation and Mitigation Strategy (REMS). Under the Tracleer REMS, prescribers, patients, and pharmacies must enroll in the program.
Hepatotoxicity:
Teratogenicity:
|
Overview
Bosentan is an endothelin receptor antagonist that is FDA approved for the {{{indicationType}}} of pulmonary arterial hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, hypotension, palpitations, flushing, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Pulmonary Arterial Hypertension
- Tracleer® is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability and to decrease clinical worsening. Studies establishing effectiveness included predominantly patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (60%), PAH associated with connective tissue diseases (21%), and PAH associated with congenital heart disease with left-to-right shunts (18%).
- Patients with WHO Class II symptoms showed reduction in the rate of clinical deterioration and a trend for improvement in walk distance. Physicians should consider whether these benefits are sufficient to offset the risk of hepatotoxicity in WHO Class II patients, which may preclude future use as their disease progresses.
- Healthcare professionals who prescribe Tracleer must enroll in the Tracleer Access Program (T.A.P.) and must comply with the required monitoring to minimize the risks associated with Tracleer.
- Dosing Information
- Initiate treatment at 62.5 mg twice daily for 4 weeks and then increase to the maintenance dose of 125 mg twice daily. Doses above 125 mg twice daily did not appear to confer additional benefit sufficient to offset the increased risk of hepatotoxicity.
- Tracleer should be administered in the morning and evening with or without food.
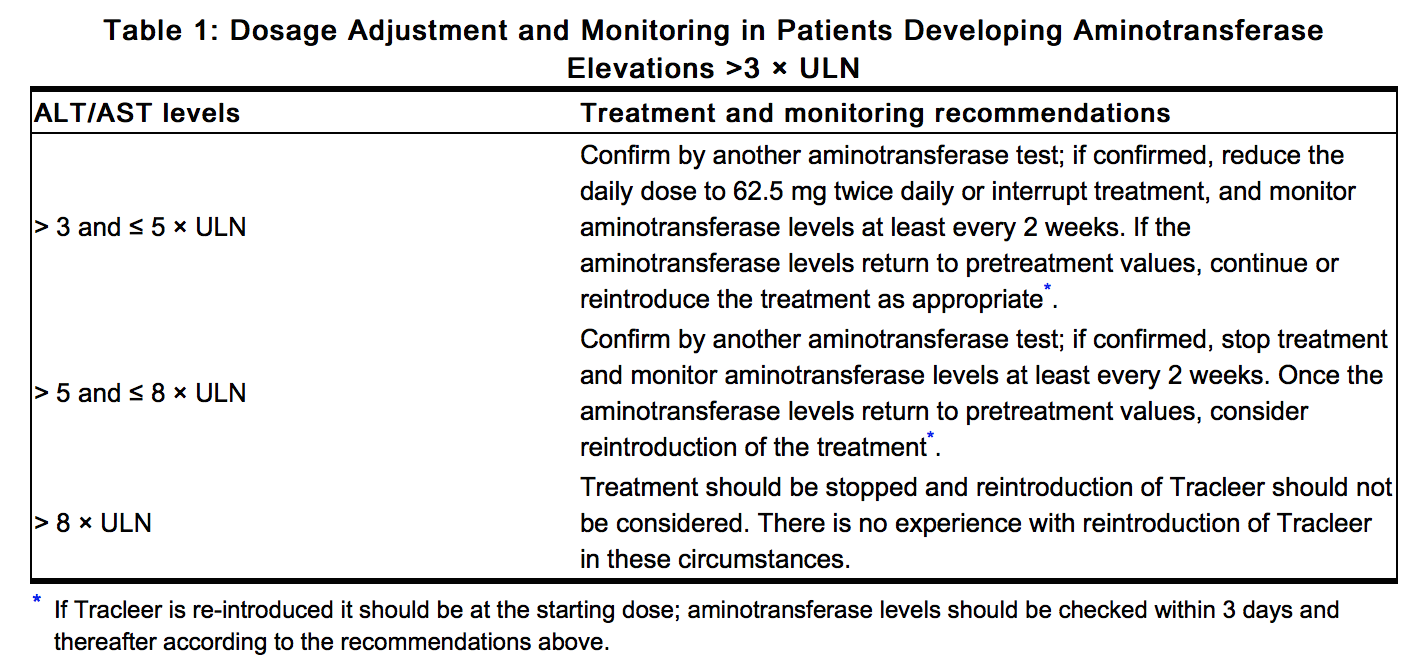
Dosage Adjustments for Patients Developing Aminotransferase Elevations
- Measure liver aminotransferase levels prior to initiation of treatment and then monthly. If aminotransferase levels increase, revise the monitoring and treatment plan. The table below summarizes the dosage adjustment and monitoring recommendations for patients who develop aminotransferase elevations >3 × ULN during therapy with Tracleer. Discontinue Tracleer if liver aminotransferase elevations are accompanied by clinical symptoms of hepatotoxicity (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 × ULN. There is no experience with the reintroduction of Tracleer in these circumstances. Information.
Patients with Low Body Weight
- In patients with a body weight below 40 kg but who are over 12 years of age, the recommended initial and maintenance dose is 62.5 mg twice daily. There is limited information about the safety and efficacy of Tracleer in children between the ages of 12 and 18 years.
- Coadministration of Tracleer in Patients on Ritonavir
- In patients who have been receiving ritonavir for at least 10 days, start Tracleer at 62.5 mg once daily or every other day based upon individual tolerability.
- Coadministration of Ritonavir in Patients on Tracleer
Use in Patients with Pre-existing Hepatic Impairment
- Tracleer should generally be avoided in patients with moderate or severe liver impairment. Initiation of Tracleer should generally be avoided in patients with elevated aminotransferases >3 × ULN. No dose adjustment is required in patients with mildly impaired liver function.
Treatment Discontinuation
- There is limited experience with abrupt discontinuation of Tracleer. No evidence for acute rebound has been observed. Nevertheless, to avoid the potential for clinical deterioration, gradual dose reduction (62.5 mg twice daily for 3 to 7 days) should be considered.
There is limited information regarding Off-Label Guideline-Supported Use of Bosentan in adult patients.
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
- Dosing Information
- 62.5 mg orally twice daily for 4 weeks, followed by 125 mg twice daily or 62.5 mg twice daily in patients weighing less than 40 kilograms.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Bosentan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Bosentan in pediatric patients.
Contraindications
- Pregnancy
- Use of Tracleer is contraindicated in females who are or may become pregnant. To prevent pregnancy, females of childbearing potential must use two reliable forms of contraception during treatment and for one month after stopping Tracleer.
- Use with Cyclosporine A
- Coadministration of cyclosporine A and bosentan resulted in markedly increased plasma concentrations of bosentan. Therefore, concomitant use of Tracleer and cyclosporine A is contraindicated.
- Use with Glyburide
- Hypersensitivity
- Tracleer is contraindicated in patients who are hypersensitive to bosentan or any component of the product. Observed reactions include rash and angioedema.
Warnings
|
WARNING: RISKS OF HEPATOTOXICITY and TERATOGENICITY
See full prescribing information for complete Boxed Warning.
* Because of the risks of hepatotoxicity and birth defects, Tracleer is available only through a restricted program called the Tracleer Access Program (T.A.P.). T.A.P. is a component of the Tracleer Risk Evaluation and Mitigation Strategy (REMS). Under the Tracleer REMS, prescribers, patients, and pharmacies must enroll in the program.
Hepatotoxicity:
Teratogenicity:
|
- Hepatotoxicity
- Elevations in ALT or AST by more than 3 × ULN were observed in 11% of Tracleer-treated patients (n = 658) compared to 2% of placebo-treated patients (n = 280). Three-fold increases were seen in 12% of 95 pulmonary arterial hypertension (PAH) patients on 125 mg twice daily and 14% of 70 PAH patients on 250 mg twice daily. Eight-fold increases were seen in 2% of PAH patients on 125 mg twice daily and 7% of PAH patients on 250 mg twice daily. Bilirubin increases to ≥3 × ULN were associated with aminotransferase increases in 2 of 658 (0.3%) of patients treated with Tracleer. The combination of hepatocellular injury (increases in aminotransferases of > 3 × ULN) and increases in total bilirubin (≥ 2× ULN) is a marker for potential serious hepatotoxicity.
- Elevations of AST or ALT associated with Tracleer are dose-dependent, occur both early and late in treatment, usually progress slowly, are typically asymptomatic, and usually have been reversible after treatment interruption or cessation. Aminotransferase elevations also may reverse spontaneously while continuing treatment with Tracleer.
- Liver aminotransferase levels must be measured prior to initiation of treatment and then monthly and therapy adjusted accordingly [see Dosage and Administration (2.2)]. Discontinue Tracleer if liver aminotransferase elevations are accompanied by clinical symptoms of hepatotoxicity (such as nausea, vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 × ULN.
- Prescribing and Distribution Program for Tracleer
- Because of the risks of hepatotoxicity and birth defects, Tracleer is available only through a restricted program called the Tracleer Access Program (T.A.P.) As a component of the Tracleer REMS, prescribers, patients, and pharmacies must enroll in the program.
- Required components of the Tracleer REMS are:
- Healthcare professionals who prescribe Tracleer must review the prescriber educational materials, enroll in T.A.P. and comply with its requirements.
- Healthcare professionals must (1) review serum aminotransferases (ALT/AST) and bilirubin, and agree to order and monitor these tests monthly; and (2) for females of childbearing potential, confirm that the patient is not pregnant, and agree to order and monitor pregnancy tests monthly.
- To receive Tracleer, all patients must understand the risks and benefits, complete a patient enrollment form, and be re-enrolled annually by their prescriber.
- Pharmacies that dispense Tracleer must enroll in the program and agree to comply with the T.A.P. requirements.
- Further information about Tracleer and T.A.P. is available at www.tracleerrems.com or 1-866-228-3546.
- Patients with Pre-existing Hepatic Impairment
- Tracleer is not recommended in patients with moderate or severe liver impairment. In addition, initiation of Tracleer should generally be avoided in patients with elevated aminotransferases (> 3 × ULN) prior to drug initiation because monitoring hepatotoxicity in these patients may be more difficult.
- Fluid Retention
- Peripheral edema is a known clinical consequence of PAH and worsening PAH and is also a known effect of Tracleer and other endothelin receptor antagonists. In PAH clinical trials with Tracleer, combined adverse events of fluid retention or edema were reported in 1.7 percent (placebo-corrected) of patients
- In addition, there have been numerous postmarketing reports of fluid retention in patients with pulmonary hypertension occurring within weeks after starting Tracleer. Patients required intervention with a diuretic, fluid management, or hospitalization for decompensating heart failure.
- If clinically significant fluid retention develops, with or without associated weight gain, further evaluation should be undertaken to determine the cause, such as Tracleer or underlying heart failure, and the possible need for treatment or discontinuation of Tracleer.
- Pulmonary Veno-Occlusive Disease
- Should signs of pulmonary edema occur, consider the possibility of associated pulmonary veno-occlusive disease and consider whether Tracleer should be discontinued.
- Decreased Sperm Counts
- Decreased sperm counts have been observed in patients receiving Tracleer. Preclinical data also suggest that Tracleer, like other endothelin receptor antagonists, may have an adverse effect on spermatogenesis.
- Decreases in Hemoglobin and Hematocrit
- Treatment with Tracleer can cause a dose-related decrease in hemoglobin and hematocrit. There have been postmarketing reports of decreases in hemoglobin concentration and hematocrit that have resulted in anemia requiring transfusion. It is recommended that hemoglobin concentrations be checked after 1 and 3 months, and every 3 months thereafter. If a marked decrease in hemoglobin concentration occurs, further evaluation should be undertaken to determine the cause and need for specific treatment.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Safety data on Tracleer were obtained from 13 clinical studies (9 placebo-controlled and 4 open-label) in 870 patients with pulmonary arterial hypertension and other diseases. Doses up to 8 times the currently recommended clinical dose (125 mg twice daily) were administered for a variety of durations. The exposure to Tracleer in these trials ranged from 1 day to 4.1 years (n=94 for 1 year; n=61 for 1.5 years and n=39 for more than 2 years). Exposure of pulmonary arterial hypertension patients (n=328) to Tracleer ranged from 1 day to 1.7 years (n=174 more than 6 months and n=28 more than 12 months).
- Treatment discontinuations due to adverse events other than those related to pulmonary hypertension during the clinical trials in patients with pulmonary arterial hypertension were more frequent on Tracleer (6%; 15/258 patients) than on placebo (3%; 5/172 patients). In this database the only cause of discontinuations > 1% and occurring more often on Tracleer was abnormal liver function.
- The adverse drug events that occurred in ≥3% of the Tracleer-treated patients and were more common on Tracleer in placebo-controlled trials in pulmonary arterial hypertension at doses of 125 or 250 mg twice daily are shown in Table 2:
Decreased Sperm Counts
- An open-label, single arm, multicenter, safety study evaluated the effect on testicular function of Tracleer 62.5 mg twice daily for 4 weeks, followed by 125 mg twice daily for 5 months. Twenty-five male patients with WHO functional class III and IV PAH and normal baseline sperm count were enrolled. Twenty-three completed the study and 2 discontinued due to adverse events not related to testicular function. There was a decline in sperm count of at least 50% in 25% of the patients after 3 or 6 months of treatment with Tracleer. Sperm count remained within the normal range in all 22 patients with data after 6 months and no changes in sperm morphology, sperm motility, or hormone levels were observed. One patient developed marked oligospermia at 3 months and the sperm count remained low with 2 follow-up measurements over the subsequent 6 weeks. Tracleer was discontinued and after 2 months the sperm count had returned to baseline levels. Based on these findings and preclinical data from endothelin receptor antagonists, it cannot be excluded that endothelin receptor antagonists such as Tracleer have an adverse effect on spermatogenesis.
Decreases in Hemoglobin and Hematocrit
- Treatment with Tracleer can cause a dose-related decrease in hemoglobin and hematocrit. It is recommended that hemoglobin concentrations be checked after 1 and 3 months, and every 3 months thereafter. If a marked decrease in hemoglobin concentration occurs, further evaluation should be undertaken to determine the cause and need for specific treatment.
- The overall mean decrease in hemoglobin concentration for Tracleer-treated patients was 0.9 g/dL (change to end of treatment). Most of this decrease of hemoglobin concentration was detected during the first few weeks of Tracleer treatment and hemoglobin levels stabilized by 4–12 weeks of Tracleer treatment. In placebo-controlled studies of all uses of Tracleer, marked decreases in hemoglobin (> 15% decrease from baseline resulting in values < 11 g/dL) were observed in 6% of Tracleer-treated patients and 3% of placebo-treated patients. In patients with PAH treated with doses of 125 and 250 mg twice daily, marked decreases in hemoglobin occurred in 3% compared to 1% in placebo-treated patients.
- A decrease in hemoglobin concentration by at least 1 g/dL was observed in 57% of Tracleer-treated patients as compared to 29% of placebo-treated patients. In 80% of those patients whose hemoglobin decreased by at least 1 g/dL, the decrease occurred during the first 6 weeks of Tracleer treatment.
- During the course of treatment the hemoglobin concentration remained within normal limits in 68% of Tracleer-treated patients compared to 76% of placebo patients. The explanation for the change in hemoglobin is not known, but it does not appear to be hemorrhage or hemolysis.
Postmarketing Experience
- There have been several postmarketing reports of angioedema associated with the use of Tracleer. The onset of the reported cases occurred within a range of 8 hours to 21 days after starting therapy. Some patients were treated with an antihistamine and their signs of angioedema resolved without discontinuing Tracleer.
- The following additional adverse reactions have been reported during the postapproval use of Tracleer. Because these adverse reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Tracleer exposure:
- Unexplained hepatic cirrhosis
- Liver failure
- Hypersensitivity
- Thrombocytopenia
- Rash
- Jaundice
- Anemia requiring transfusion
- Neutropenia and leukopenia
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bosentan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bosentan during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Bosentan with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Bosentan with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Bosentan with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Bosentan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Bosentan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Bosentan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Bosentan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bosentan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bosentan in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Bosentan in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Bosentan in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Bosentan in the drug label.
Pharmacology
| |
Bosentan
| |
| Systematic (IUPAC) name | |
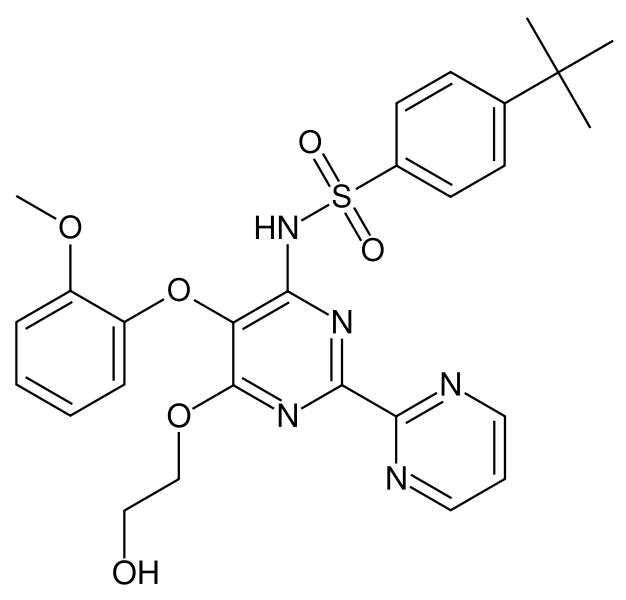
| N-[6-(2-hydroxyethoxy)-5- (2-methoxyphenoxy) -2-pyrimidin-2-yl-pyrimidin-4-yl] -4-tert- butyl-benzenesulfonamide | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 551.615 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 50% |
| Protein binding | >98% |
| Metabolism | Hepatic |
| Half life | 5 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
X |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Bosentan in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Bosentan in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Bosentan in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Bosentan in the drug label.
How Supplied
Storage
There is limited information regarding Bosentan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Bosentan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Bosentan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Bosentan in the drug label.
Precautions with Alcohol
- Alcohol-Bosentan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Tracleer®[2]
Look-Alike Drug Names
- Tracleer® — Tricor®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Jaïs, Xavier (2008-12-16). "Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial". Journal of the American College of Cardiology. 52 (25): 2127–2134. doi:10.1016/j.jacc.2008.08.059. ISSN 1558-3597. PMID 19095129. Unknown parameter
|coauthors=ignored (help) - ↑ "TRACLEER (bosentan) tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Bosentan |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Bosentan |Label Name=Bosentan11.png
}}
{{#subobject:
|Label Page=Bosentan |Label Name=Bosentan11.png
}}