Calcium lactate gluconate: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
m (Protected "Calcium lactate gluconate": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 35: | Line 35: | ||
}} | }} | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} | ||
==Overview== | ==Overview== | ||
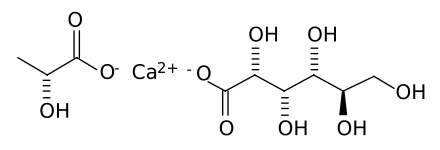
'''Calcium lactate gluconate''', also known as GLOCAL, is a soluble [[salt]] of [[calcium]], [[lactic acid]] and [[gluconic acid]] used in [[effervescent]] calcium tablets.<ref name="AustriaCodex">{{cite book|title=Austria-Codex|editor=Haberfeld, H|publisher=Österreichischer Apothekerverlag|location=Vienna|year=2009|edition=2009/2010|isbn=3-85200-196-X|language=German}}</ref> Its [[chemical formula]] is Ca<sub>5</sub>(C<sub>3</sub>H<sub>5</sub>O<sub>3</sub>)<sub>6</sub>.(C<sub>6</sub>H<sub>11</sub>O<sub>7</sub>)<sub>4</sub>.2H<sub>2</sub>O. It was first developed by [[Sandoz]], [[Switzerland]]. Calcium lactate gluconate is used in the functional and fortified food industry due to its good solubility and neutral taste.<ref>{{cite journal|author=Gerhard Gerstner |title=Calcium Lactate Gluconate – the innovative solution for extra calcium |journal=Innovations in Food Technology |volume=3 |year=2002 |pages=2–3 |url=http://www.jungbunzlauer.com/media/uploads/pdf/Special_Salts/Calcium_Lactate_Gluconate_Aug02.pdf}}</ref> In addition, it is used in various [[spherification]] techniques in [[molecular gastronomy]]. It can also be used to help neutralize HF ([[hydrofluoric acid]]) poisoning. | '''Calcium lactate gluconate''', also known as GLOCAL, is a soluble [[salt]] of [[calcium]], [[lactic acid]] and [[gluconic acid]] used in [[effervescent]] calcium tablets.<ref name="AustriaCodex">{{cite book|title=Austria-Codex|editor=Haberfeld, H|publisher=Österreichischer Apothekerverlag|location=Vienna|year=2009|edition=2009/2010|isbn=3-85200-196-X|language=German}}</ref> Its [[chemical formula]] is Ca<sub>5</sub>(C<sub>3</sub>H<sub>5</sub>O<sub>3</sub>)<sub>6</sub>.(C<sub>6</sub>H<sub>11</sub>O<sub>7</sub>)<sub>4</sub>.2H<sub>2</sub>O. It was first developed by [[Sandoz]], [[Switzerland]]. Calcium lactate gluconate is used in the functional and fortified food industry due to its good solubility and neutral taste.<ref>{{cite journal|author=Gerhard Gerstner |title=Calcium Lactate Gluconate – the innovative solution for extra calcium |journal=Innovations in Food Technology |volume=3 |year=2002 |pages=2–3 |url=http://www.jungbunzlauer.com/media/uploads/pdf/Special_Salts/Calcium_Lactate_Gluconate_Aug02.pdf}}</ref> In addition, it is used in various [[spherification]] techniques in [[molecular gastronomy]]. It can also be used to help neutralize HF ([[hydrofluoric acid]]) poisoning. | ||
==References== | ==References== | ||
| Line 48: | Line 45: | ||
{{Mineral supplements}} | {{Mineral supplements}} | ||
[[Category:Calcium compounds]] | [[Category:Calcium compounds]] | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 18:31, 18 August 2015
Template:Chembox E numberTemplate:Chembox AppearanceTemplate:Chembox SolubilityInWater
| |
| Names | |
|---|---|
| IUPAC name
calcium; (R/S)-2-hydroxypropanoate; (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| |
| |
| Properties | |
| C9H16CaO10 | |
| Molar mass | 324.30 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Calcium lactate gluconate, also known as GLOCAL, is a soluble salt of calcium, lactic acid and gluconic acid used in effervescent calcium tablets.[1] Its chemical formula is Ca5(C3H5O3)6.(C6H11O7)4.2H2O. It was first developed by Sandoz, Switzerland. Calcium lactate gluconate is used in the functional and fortified food industry due to its good solubility and neutral taste.[2] In addition, it is used in various spherification techniques in molecular gastronomy. It can also be used to help neutralize HF (hydrofluoric acid) poisoning.
References
- ↑ Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- ↑ Gerhard Gerstner (2002). "Calcium Lactate Gluconate – the innovative solution for extra calcium" (PDF). Innovations in Food Technology. 3: 2–3.