Structural phosphate
Editor-In-Chief: Henry A. Hoff
Overview
Inside a cell, phosphate may be structural to a nucleic acid such as DNA and RNA or phospholipid. Outside the cell, phosphate may be dissolved in extracellular fluid (ECF) or form structures such as bone and teeth. Bringing phosphate in any form into the cell from a phosphate containing structure or for such a structure and when needed transporting phosphate out of the cell perhaps to a structure is a necessary activity of phosphate homeostasis for that cell.
Introduction
Inorganic phosphate (Pi) (structural phosphate) behaves as a serine phosphate and is not the same as the enzyme-bound phosphate (catalytic phosphate) derived from ATP during a catalytic reaction.[1] The catalytic phosphate in a nucleotide is usually the phosphate farthest from the nucleoside. The structural phosphate becomes the hydrolyzed nucleotide after the catalysis that associates with the binding of the polymer. The structural phosphate is a part of each polymer whether it is a microfilament, microtubule, phospholipid, or nucleic acid. Inorganic phosphates such as Pi and PPi can be precipitated with divalent cations like Ca2+, Mg2+, or others, e.g. Mn2+, to form a variety of tissue and mineral composites: cartilage, teeth, and bone. These in turn eventually become localized to the natural environment and form phosphorites.
Catalytic phosphate
Inorganic phosphate (Pi) (structural phosphate) behaves as a serine phosphate and is not the same as the enzyme-bound phosphate (catalytic phosphate) derived from ATP during a lyase reaction of EC 4.1.3.8.[1] Evidence indicates that EC 4.1.3.8 contains one structural phosphate for each catalytic phosphate, which does not affect its catalytic activity.[1] The γ phosphate of ATP is the catalytic phosphate. EC 4.1.3.8 ATP citrate lyase contains acid-labile, catalytic phosphate after the enzyme is reacted with ATP and Mg2+, whereas the structural phosphate is acid-stable, base-labile.[2] There are 2 mol of acid-labile catalytic phosphate and 2 mol of base-labile structural phosphate per tetramer. A pH 7.5 buffer associated with EC 4.1.3.8 contains concentrations of ADP, Pi, and free Mg2+ similar to those found in vivo.[2] Glucagon induces an incorporation of acid-stable phosphate into ATP citrate lyase without a concomitant change in enzyme activity.[2]
Intracellular structural phosphate
In the mathematical modeling of cell growth and phosphatase biosynthesis it is necessary to determine the intracellular concentration of structural phosphate.[3]
Nucleotides
Analysis of the phosphate moiety present in mononucleotides (NMP) can allow the identification of structural phosphate binding motifs.[4]
When nucleotide hydrolysis is coupled to the polymerization process, the two ends of the polymer behave differently, and the critical concentration required for assembly at one end is different from that required for the opposite end. However, NTP hydrolysis is usually associated but not directly coupled with polymerization. When there are NTP-bound subunits at the ends, the polymer is stable and continues to grow slowly. When at the polymer ends there are subunits containing NDP, the polymer is in a shrinking phase, depolymerizes rapidly and completely, and much faster at lower mer concentrations.
Microfilaments
During the assembly of actin into microfilaments, actin-ATP becomes actin ADP upon polymerization.
Microtubules
Pi, BeF3 or AIF4, structural phosphate analogs, have a stabilizing effect on microtubules containing GDP tubulin.[5] The structural phosphate of microtubules is the GDP in the interior of the polymer.
Phospholipid
Phospholipids are a major component of the plasma membrane and organelle membranes. In normal cell plasma membranes, phospholipids are asymmetrically distributed: phosphatidylcholine (PC) and sphingomyelin (SM) predominantly in the exoplasmic leaflet and phosphatidylserine (PS) and phosphatidylethanolamine (PE) in the cytoplasmic leaflet. All phospholipids in eukaryotic plasma membranes undergo a slow passive transbilayer movement.[6] Half-times of redistribution are 3.6 min for PS.[6] Both PC and SM have higher diffusion rates probably indicative of endocytosis.
A typical molecule of PC, SM, PS, or PE contains one monophosphate group. The entire PC molecule contains glycerol, Pi, choline and fatty acids for 760 Da[7] with an average diameter of ~2.16 nm. However, the fatty acids extend between the bilayers of the cell membrane. The head of a PC molecule is 314 Da[8], with an average diameter of ~1.62 nm. The head contains the structural monophosphate. For an cell of 10 µm diameter, there may be up to ~7.6 x 107 molecules of PC in the exoplasmic leaflet, if the leaflet were entirely composed of PC.
The sphingomyelins (SMs) are found largely in the brain and other nervous tissue. They contain phosphocholine or phosphoethanolamine as their polar head group. The head group containing phosphocholine is 285 Da[9], with an average diameter of ~1.56 nm. SMs can have fatty acid tails, such as in sphingosine phosphorylcholine 465 Da[10] (one tail) or TNPAL-sphingomyelin 874 Da[11] (two tails). Although the head size of the SMs is comparable to that of PC, the number of phosphates in the exoplasmic leaflet would be at ~8.2 x 107, if composed entirely of SM.
In the cytoplasmic leaflet are PS and PE. The head of PS is 385 Da[12], with an average diameter of ~1.72 nm. As the cell membrane averages about 7 nm in thickness, the cytoplasmic leaflet can have ~6.7 x 107 molecules of PS, if totally composed of PS. The head of PE is 271 Da[13], with an average diameter of ~1.54 nm. The cytoplasmic leaflet would have ~8.4 x 107 molecules of PE, if totally composed of PE.
If half and half, the number of phosphates in the cytoplasmic leaflet would be ~7.6 x 107. Half and half in the exoplasmic leaflet would yield ~7.9 x 107 molecules of phosphate. The entire cell membrane could have up to ~1.6 x 108 molecules of phosphate.
Nucleic acid
Phosphate is a component of DNA and RNA. Most of the structural phosphate in nucleic acids is in the phospho-diester linkage.[14]
Ribonucleic acid (RNA)
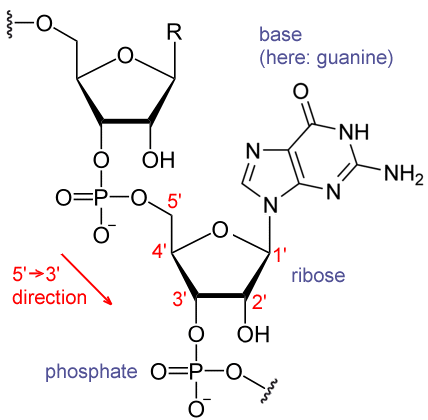
The variety and type of RNA is extensive. Each has to be transcribed from the applicable portion of DNA in the euchromatin. The unfolded structure of euchromatin allows gene regulatory proteins and RNA polymerase (RNAP) complexes to bind to the DNA sequence, which can then initiate the transcription process. Control of the process of gene transcription affects patterns of gene expression and thereby allows a cell to adapt to a changing environment, perform specialized roles within an organism, and maintain basic metabolic processes necessary for survival. RNAP can initiate transcription at specific DNA sequences known as promoters. It then produces an RNA chain which is complementary to the template DNA strand. The process of adding nucleotides to the RNA strand is known as elongation.
| Type | Abbr. | Function | Distribution | Ref. |
|---|---|---|---|---|
| Messenger RNA | mRNA | Codes for protein | All cells | |
| Ribosomal RNA | rRNA | Translation | All cells | |
| Signal recognition particle RNA | 7SL RNA or SRP RNA | Membrane integration / mRNA tagging for export | All organisms | [15] |
| Transfer RNA | tRNA | Translation | All cells | |
| Transfer-messenger RNA | tmRNA | Rescuing stalled ribosomes Terminating translation | Bacteria | [16] |
| Type | Abbr. | Function | Distribution | Ref. |
|---|---|---|---|---|
| Small nuclear RNA | snRNA | Splicing and other functions | Eukaryotes and archaea | [17] |
| Small nucleolar RNA | snoRNA | Nucleotide modification of RNAs RNA editing | Eukaryotes and archaea | [18] |
| SmY RNA | SmY | mRNA trans-splicing | Nematodes | [19] |
| Small Cajal body-specific RNA | scaRNA | Type of snoRNA; Nucleotide modification of RNAs | ||
| Guide RNA | gRNA | mRNA nucleotide modification / RNA editing | Kinetoplastid mitochondria | [20] |
| Ribonuclease P | RNase P | tRNA maturation | All organisms | [21] |
| Ribonuclease MRP | RNase MRP | rRNA maturation, DNA replication | Eukaryotes | [22] |
| Y RNA | RNA processing, DNA replication | Animals | [23] | |
| Telomerase RNA | Telomere synthesis | Most eukaryotes | [24] | |
| Ribozyme | Catalysis | All cells | ||
| Transposon | Self-propagating | All cells |
| Type | Abbr. | Function | Distribution | Ref. |
|---|---|---|---|---|
| Antisense RNA | aRNA | Transcriptional attenuation / mRNA degradation / mRNA stabilisation / Translation block Gene regulation | All organisms | [25][26] |
| Cis-natural antisense transcript | Gene regulation | |||
| CRISPR RNA | crRNA | Resistance to parasites, probably by targeting their DNA | Bacteria and archaea | [27] |
| Long noncoding RNA | Long ncRNA | Various | Eukaryotes | |
| MicroRNA | miRNA | Gene regulation | Most eukaryotes | [28] |
| Piwi-interacting RNA | piRNA | Transposon defense Gene regulation | Animal germline cells | [29][30] |
| Small interfering RNA | siRNA | Gene regulation | Most eukaryotes | [31] |
| Trans-acting siRNA | tasiRNA | Gene regulation | Land plants | [32] |
| Repeat associated siRNA | rasiRNA | Type of piRNA; transposon defense | Drosophila | [33] |
Enstructuring Pi into any RNA is usually not accomplished by reacting directly with ribose for RNA, deoxyribose present in DNA, purines, or pyrimidines. Although DNA records the sequence of nucleobases that are connected by ribose to Pi, the transcription process chosen by probably all life on Earth requires triphosphate nucleotides (NTPs).
For example, consider making a mRNA for a particular protein from the point of view of phosphate enstructuring. As of 2008 dystrophin has the longest gene known, at locus Xp21.2. The primary transcript measures 2.4 megabases (thus the gene comprises 0.008% of the human genome), and takes 16 hours to transcribe. The 79 exons[34] code for a protein of over 3500 amino acid (aa) residues. It is human GeneID: 1756.[35] Isoform Dp427c has 3677 aa[36] which are specified by 3677 codons of 3 nucleotides each. That's 11,031 NMPs with one Pi each.
But the gene portion that is transcribed also contains a 5' cap requiring one GTP, a 5' UTR, 78 introns, and a 3' UTR or as mentioned 2.4 Mb of one Pi each. In this excess generation of RNA dystrophin is not unique. More than 95% of the enstructured phosphate for RNA synthesized by RNA polymerase II never reaches the cytoplasm. The main reason for this is due to the removal of introns which account for 80% of the total bases.[37]
Per the apparently available phosphate reserve in a cell or intracellular phosphate of about 10-3 Pi (1 molecule of Pi per 1000 other molecules, such as water) at any particular location and a rate of about 42 NTPs per second to transcribe this mRNA, some form of intranuclear transport other than diffusion is needed. While the number of transcription units varies at any given moment, should per transcription factories there be ~64,000 polymerase II active transcription units operating at about 42 NTPs per second, there would need to be a way to supply a total phosphate of ~2,700,000 NTPs per second to all transcription units in parallel.
With respect to phosphate reserves each ATP is recycled about once every minute on average. This may also be true for CTP, GTP, and UTP. That is ~0.07 recycled NTPs per second are available to transcribe on average.
Using the information derived regarding signal transduction for a water molecule of speed 15 µms-1, if a nucleus of 5 µm diameter contains ~64,000 transcription units uniformly distributed throughout (a volume with ~120 nm diameter for each unit), it would take about 40 seconds for a GTP molecule to interact with one unit with ten molecules in each unit volume. The GTP molecule would have a speed of ~2800 nms-1. But to get to 24 ms between interactions would take ~17,000 NTP molecules in a volume of ~120 nm diameter. That is ~68 µg/ml GTP. As the total number of normally available intracellular phosphate molecules, if uniformly distributed throughout the cell, would be ~33,000 molecules of all phosphate compunds within each ~120 nm diameter volume, diffusion may be sufficient. Pulling in additional phosphate from extracellular fluid would help to increase the amount of available phosphate, probably enough to make 42 NTPs per second reasonable.
Subtracting the estimated number of phosphates in the genome DNA yields ~21,000 phosphate molecules for all other purposes. The amount of phosphate in the cell membrane would reduce the available phosphate to ~20,000 molecules. But, if half the available phosphate is already tied up in RNA or other structures, then transport across the cell membrane and diffusion throughout the cell is mandatory.
Alternatively, if the actual translational speed of a water molecule is closer to 75 µms-1, the needed phosphate is in ~3,400 NTPs. However, for transcription of a mRNA roughly equal amounts of ATP, CTP, GTP, and UTP need to be present in each ~120 nm diameter volume. That's ~13,600 NTPs with 3 phosphates each for ~41,000 phosphates. To have the needed concentration in the nucleus, the entire cell needs to be at the same level of phosphate in the cytosol. The total phosphate uniformly distributed by diffusion throughout the cytosol is ~24 x 109: ~6.0 x 109 each of ATP, CTP, GTP, and UTP.
| Abbr. | GeneID | Number of basepairs | Accumulative average phosphates |
|---|---|---|---|
| mRNA | 1 | 1488 | 1488 |
| mRNA | 2 | 4425 | 2956.5 |
| mRNA | 9 | 873 | 2262 |
| mRNA | 10 | 873 | 1914.8 |
| mRNA | 12 | 1272 | 1786.2 |
| ncRNA | 245 | 2768 | 2768 |
| ncRNA | 303 | 1343 | 2055.5 |
| ncRNA | 305 | 1350 | 1820.3 |
| ncRNA | 1057 | 985 | 1611.5 |
| ncRNA | 1197 | 1812 | 1651.6 |
| ncRNA | 1587 variant 1 | 2642 | 1816.7 |
| ncRNA | 1587 variant 2 | 2396 | 1988.4 |
| ncRNA | 1587 variant 3 | 2491 | 1973.4 |
| miscRNA (ncRNA) | 711 | 2264 | 2264 |
| miscRNA (ncRNA) | 3262 | 1198 | 1731 |
| miscRNA (ncRNA) | 5820 | 1918 | 1793.3 |
| miscRNA (Long? ncRNA) | 7503 | 19271 | 6162.8 |
| miscRNA (ncRNA) | 8123 | 3373 | 5604.8 |
| miscRNA (ncRNA) | 8420 | 939 | 4827.2 |
| rRNA | 4549 | 953 | 953 |
| rRNA | 4550 | 1558 | 1255.5 |
| rRNA (ribosome component) | 6052 | --- | --- |
| rRNA (ribosome component) | 6053 | --- | --- |
| rRNA (ribosome component) | 6054 | --- | --- |
| rRNA (ribosome component) | 6055 | --- | --- |
| rRNA (ribosome component) | 6056 | --- | --- |
| rRNA | 100008587 | 156 | 889 |
| rRNA | 100008588 | 1869 | 1134 |
| rRNA | 100008589 | 5070 | 1921.2 |
| SRP RNA (miscRNA) | 6029 | 299 | 299 |
| SRP RNA (scRNA) | 378706 | 299 | 299 |
| tRNA | 4511 (mitochondrial) | 65 | 65 |
| tRNA | 4553 (mitochondrial) | 68 | 66.5 |
| tRNA | 4555 (mitochondrial) | 67 | 66.7 |
| tRNA | 4556 (mitochondrial) | 68 | 67 |
| tRNA | 4558 (mitochondrial) | 70 | 67.6 |
| snRNA (spliceosome) | 6060 | 164 | 164 |
| snRNA (spliceosome) | 6066 | 188 | 176 |
| snRNA (ncRNA) | 125050 | 332 | 228 |
| snRNA (spliceosome) | 100151683 | 130 | 203.5 |
| snRNA (spliceosome) | 100151684 | 125 | 187.8 |
| snoRNA (ncRNA) | 6043 | 135 | 135 |
| snoRNA (ncRNA) | 6044 | 154 | 144.5 |
| snoRNA (ncRNA) | 6079 | 148 | 145.7 |
| snoRNA (ncRNA) | 6080 | 207 | 161 |
| snoRNA (ncRNA) | 6081 | 205 | 169.8 |
| RNase P (miscRNA) | 85495 | 341 | 341 |
| RNase MRP (miscRNA) | 6023 | 265 | 265 |
| Y RNA (scRNA) | 6084 | 113 | 113 |
| Y RNA (scRNA) | 6085 | 102 | 107.5 |
| Y RNA (scRNA) | 6086 | 96 | 103.7 |
| Y RNA (scRNA) | 6090 | 84 | 98.8 |
| Telomerase RNA (miscRNA) | 7012 | 451 | 451 |
| Ribozyme (ncRNA | 54677 | 1264 | 1264 |
| Alu sequence (Transposon) | ? | ~300 | ~300 |
| aRNA (miscRNA) | 6315 | 1472 | 1472 |
| aRNA (miscRNA) | 8475 | 1286 | 1379 |
| aRNA (miscRNA) | 9383 | 37027 | 13261.7 |
| aRNA (miscRNA) | 10108 | --- | 13261.7 |
| aRNA (miscRNA) | 10737 variant 1 | 1357 | 13261.7 |
| aRNA (miscRNA) | 10737 variant 2 | 1115 | 8451.4 |
| aRNA (miscRNA) | 10740 | 5125 | 7897 |
| Long ncRNA | 7503 | 19271 | 19271 |
| Long ncRNA | 9383 | 37027 | 28149 |
| Long ncRNA | 10984 | 59461 | 38586.3 |
| miRNA | 406991 | 71 | 71 |
| miRNA | 406952 | 83 | 77 |
| miRNA | 406902 | 109 | 87.7 |
| miRNA | 407043 | 109 | 93 |
| miRNA | 407006 | 109 | 96.2 |
| piRNA | ? | 29-30 | 29-30 |
| siRNA | ? | 20-25 | 20-25 |
In the table above those RNAs with a GeneID less than 30000 are found by searching among the human GeneIDs up to 30000. A total of 230 RNA genes, including variants, occur prior to GeneID 30000. There are as of July 22, 2009, 15827 human genes of all types, including variants and pseudogenes, at and before GeneID: 30000. Of the pseudogenes, 521 are inactive. Many of the active pseudogenes are RNA transcribing genes. Some 611 of these GeneIDs are also not transcribed genes. Some 14,695 are active and transcribed.
Using a likely cellular phosphate budget in a human adult as a guide, an average cell could be operating ~28,800 transcription units. For a lateral speed of 37 µms-1 of a water molecule, an average cell could operate ~14,400 transcription units by diffusion.
For an average cell of "default" cell type running the 14,695 active and transcribeable genes including variants, 14,464 mRNAs and 230 other assorted RNAs are being transcribed. As the above table lists the number of phosphates per RNA on average, an RNA transcription budget can be estimated, assuming each is halfway through transcription.
| Abbr. | Number of RNAs | Number of structural phosphates | Accumulative phosphates |
|---|---|---|---|
| mRNA | 14,464 | ~12.9 x 106 | ~12.9 x 106 |
| snoRNA | 77 | ~6500 | ~12.9 x 106 |
| tRNA | 59 | ~2000 | ~12.9 x 106 |
| miscRNA | 39 | ~94,000 | ~13.0 x 106 |
| ncRNA | 21 | ~21,000 | ~13.0 x 106 |
| RNA | 11 | ~10,000 | ~13.1 x 106 |
| aRNA | 7 | ~28,000 | ~13.1 x 106 |
| rRNA | 6 | ~5800 | ~13.1 x 106 |
| Y RNA | 4 | ~200 | ~13.1 x 106 |
| SRP | 2 | ~300 | ~13.1 x 106 |
| snRNA | 2 | ~200 | ~13.1 x 106 |
| Long ncRNA | 2 | ~39,000 | ~13.1 x 106 |
Deoxyribonucleic acid (DNA)
The entire human genome has ~3.4 Gb in its DNA, with one Pi each. That is ~3.4 x 109 Pi tied up in DNA per strand, ~6.8 x 109 Pi total structural phosphate in each nucleus. With ~19 x 109 Pi per cell in some form, that leaves ~12 x 109 Pi per cell for all other functions and forms.
Mitochondria have their own genetic material, and the machinery to manufacture their own RNAs and proteins. A published human mitochondrial DNA sequence revealed 16,569 base pairs encoding 37 total genes, 24 tRNA and rRNA genes and 13 peptide genes.[38] The mitochondrial matrix contains several (2-10) copies of the mitochondrial DNA genome. Up to ~2000 mitochondria can occur per cell. This can enstructure up to ~3.3 x 108 phosphates.
Cartilage
Mineralization in the extracellular matrix starting with hydroxyapatite deposition into a collagenous scaffolding of cartilage, cementum, dentin and bone is initially regulated locally by calcium, orthophosphate, and pyrophosphate ion concentrations.
Although cuboid calcium phosphate crystals are absent in young articular cartilage, they are sometimes present in old normal articular cartilage occasionally adjacent to larger areas of pyrophosphate crystals.[39]
Endochondrial ossification of cartilage development ultimately leads to mineralization of the extracellular matrix.[40] Metabolism of Ca2+ and Pi by cartilage growth plate chondrocytes, involved in matrix vesicle formation, clearly are integral features of endochondrial calcification.[40] This calcification is apparently due to the diffusion barrier and avascularity progressing from the zone of proliferation to the zone of hypertrophy of the cartilage growth plate chondrocytes environment.[40]
Teeth
Hydroxyapatite, which is a crystalline calcium phosphate is the primary mineral of enamel.[41]
By weight, seventy percent of dentin consists of the mineral, hydroxylapatite, twenty percent is organic material, and ten percent is water.[42]
Cementum is a specialized bony substance covering the root of a tooth, composed of approximately 45% inorganic material (mainly hydroxyapatite), 33% organic material (mainly collagen) and 22% water.
Bone
During bone resorption high levels of phosphate are released into the ECF as osteoclasts tunnel into mineralized bone, breaking it down and releasing phosphate, that results in a transfer of phosphate from bone fluid to the blood. During childhood, bone formation exceeds resorption, but as the aging process occurs, resorption exceeds formation.
Transphosphorylation between nucleotides and hydroxyapatite (HA) results in a pyrophosphate on HA that is distinctive from pyrophosphate absorbed onto HA from solution. This may be due to a different orientation of the pyrophosphate on the surface depending on the origin of the pyrophosphate.[43]
Sedimentary phosphorites
Phosphogenesis under palaeoceanographic conditions that were different from modern phosphorite depositional systems occurred across a broad range of palaeoenvironments in the shallow and broad epicontinental Phosphoria Sea.[44] These conditions were revealed by oxygen isotope analyses of the structural phosphate in sedimentary phosphorites of the Upper Permian Phosphoria Formation.[44]
Acknowledgements
The content on this page was first contributed by: Henry A. Hoff.
Initial content for this page in some instances came from Wikipedia.
References
- ↑ 1.0 1.1 1.2 Linn TC, Srere PA (1979). "Identification of ATP citrate lyase as a phosphoprotein". J Biol Chem. 254 (5): 1691–8. PMID 762167. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 2.2 Janski AM, Srere PA, Cornell NW, Veech RL (1979). "Phosphorylation of ATP Citrate Lyase in Response to Glucagon". J Biol Chem. 254 (19): 9365–8. PMID 489538. Unknown parameter
|month=ignored (help) - ↑ Toda K, Yabe I (2004). "Mathematical model of cell growth and phosphatase biosynthesis in Saccharomyces carlsbergensis under phosphate limitation". Biotech Bioeng. 21 (3): 487–502. doi:10.1002/bit.260210310. Unknown parameter
|month=ignored (help) - ↑ Babor M, Soboleva V, Edelman M (2002). "Conserved Positions for Ribose Recognition: Importance of Water Bridging Interactions Among ATP, ADP and FAD-protein Complexes". J Mol Biol. 323 (3): 523–32. doi:10.1016/S0022-2836(02)00975-0. Unknown parameter
|month=ignored (help) - ↑ Avila J (1990). "Microtubule dynamics". FASEB J. 4 (15): 3284–90. PMID 2253844. Unknown parameter
|month=ignored (help) - ↑ 6.0 6.1 Pomorski T, Muller P, Zimmermann B, Burger K, Devaux PF, Herrmann A (1996). "Transbilayer movement of fluorescent and spin-labeled phospholipids in the plasma membrane of human fibroblasts: a quantitative approach". J Cell Sci. 109 (Pt 3): 687–98. PMID 8907713. Unknown parameter
|month=ignored (help) - ↑ "1-palmitoyl-2-oleoylphosphatidylcholine - PubChem Public Chemical Database".
- ↑ "Phosphatidylcholines - PubChem Public Chemical Database".
- ↑ "Sphingomyelins - PubChem Public Chemical Database".
- ↑ "sphingosine phosphorylcholine - PubChem Public Chemical Database".
- ↑ "TNPAL-Sphingomyelin - PubChem Public Chemical Database".
- ↑ "Phosphatidylserine - PubChem Public Chemical Database".
- ↑ "Phosphatidylethanolamines - PubChem Public Chemical Database".
- ↑ Battley EH (1992). "On the enthalpy of formation of Escherichia coli K-12 cells". Biotech Bioeng. 39 (1): 5–12. doi:10.1002/bit.260390103. Unknown parameter
|month=ignored (help) - ↑ Gribaldo S, Brochier-Armanet C (2006). "The origin and evolution of Archaea: a state of the art". Philos Trans R Soc Lond B Biol Sci. 361 (1470): 1007–22. doi:10.1098/rstb.2006.1841. PMID 16754611.
- ↑ Gillet R, Felden B (2001). "Emerging views on tmRNA-mediated protein tagging and ribosome rescue". Molecular Microbiology. 42 (4): 879–85. doi:10.1046/j.1365-2958.2001.02701.x.
- ↑ Thore S, Mayer C, Sauter C, Weeks S, Suck D (2003). "Crystal Structures of the Pyrococcus abyssi Sm Core and Its Complex with RNA". J. Biol. Chem. 278 (2): 1239–47. doi:10.1074/jbc.M207685200.
- ↑ Kiss T (2001). "Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs". The EMBO Journal. 20: 3617–22. doi:10.1093/emboj/20.14.3617.
- ↑ Jones TA, Otto W, Marz M, Eddy SR, Stadler PF (2009). "A survey of nematode SmY RNAs". RNA Biol. 6 (1): 5–8. PMID 19106623.
- ↑ Alfonzo JD, Thiemann O, Simpson L (1997). "The mechanism of U insertion/deletion RNA editing in kinetoplastid mitochondria". Nucleic Acids Research. 25 (19): 3751–59. doi:10.1093/nar/25.19.3751. PMID 9380494.
- ↑ Pannucci JA, Haas ES, Hall TA, Harris JK, Brown JW (1999). "RNase P RNAs from some Archaea are catalytically active". Proc Natl Acad Sci USA. 96 (14): 7803–08. doi:10.1073/pnas.96.14.7803. PMID 10393902.
- ↑ Woodhams MD, Stadler PF, Penny D, Collins LJ (2007). "RNase MRP and the RNA processing cascade in the eukaryotic ancestor". BMC Evolutionary Biology. 7: S13. doi:10.1186/1471-2148-7-S1-S13.
- ↑ Perreault J, Perreault J-P, Boire G (2007). "Ro-associated Y RNAs in metazoans: evolution and diversification". Molecular Biology and Evolution. 24 (8): 1678–89. doi:10.1093/molbev/msm084.
- ↑ Lustig AJ (1999). "Crisis intervention: The role of telomerase". Proc Natl Acad Sci USA. 96 (7): 3339–41. doi:10.1073/pnas.96.7.3339. PMID 10097039.
- ↑ Brantl S (2002). "Antisense-RNA regulation and RNA interference". Biochimica et Biophysica Acta. 1575 (1–3): 15–25. PMID 12020814.
- ↑ Brantl S (2007). "Regulatory mechanisms employed by cis-encoded antisense RNAs". Curr Opin Microbiol. 10 (2): 102–9. doi:10.1016/j.mib.2007.03.012. PMID 17387036.
- ↑ Brouns SJ, Jore MM, Lundgren M; et al. (2008). "Small CRISPR RNAs guide antiviral defense in prokaryotes". Science (New York, N.Y.). 321 (5891): 960–4. doi:10.1126/science.1159689. PMID 18703739. Unknown parameter
|month=ignored (help) - ↑ Lin S-L, Miller JD, Ying S-Y (2006). "Intronic microRNA (miRNA)". Journal of Biomedicine and Biotechnology. 2006: 1–13. doi:10.1155/JBB/2006/26818. PMID 17057362.
- ↑ Horwich MD, Li C Matranga C, Vagin V, Farley G, Wang P, Zamore PD (2007). "The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC". Current Biology. 17: 1265–72. doi:10.1016/j.cub.2007.06.030.
- ↑ Ghildiyal M, Zamore PD (2009). "Small silencing RNAs: an expanding universe". Nat. Rev. Genet. 10 (2): 94–108. doi:10.1038/nrg2504. PMID 19148191. Unknown parameter
|month=ignored (help) - ↑ Ahmad K, Henikoff S (2002). "Epigenetic consequences of nucleosome dynamics". Cell. 111 (3): 281–84. doi:10.1016/S0092-8674(02)01081-4.
- ↑ Vazquez F, Vaucheret H (2004). "Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs". Mol. Cell (16): 1–13. PMID 17057362.
- ↑ Desset S, Buchon N, Meignin C, Coiffet M, Vaury C (2008). "In Drosophila melanogaster the COM locus directs the somatic silencing of two retrotransposons through both Piwi-dependent and -independent pathways". PLoS ONE. 3 (2): e1526. doi:10.1371/journal.pone.0001526. PMC 2211404. PMID 18253480.
- ↑ Strachan T, Read AP (1999). Human molecular genetics 2 (2nd ed.). New York, USA: John Wiley & Sons, Inc. ISBN 0471133736.
- ↑ "Entrez Gene: DMD dystrophin".
- ↑ "Entrez Protein - dystrophin Dp427c isoform [Homo spiens]".
- ↑ Jackson DA, Pombo A, Iborra F (2000). "The balance sheet for transcription: an analysis of nuclear RNA metabolism in mammalian cells". FASEB J. 14 (2): 242–54. PMID 10657981.
- ↑ Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR; et al. (1981). "Sequence and organization of the human mitochondrial genome". Nature. 410 (5806): 141. doi:10.1038/290457a0. Unknown parameter
|month=ignored (help) - ↑ Ali SY, Griffiths S (1983). "Formation of calcium phosphate crystals in normal and osteoarthritic cartilage". Ann Rheum Dis. 42 (Suppl 1): 45–8. PMID 6615026. Unknown parameter
|month=ignored (help) - ↑ 40.0 40.1 40.2 Wuthier RE (1993). "Involvement of cellular metabolism of calcium and phosphate in calcification of avian growth plate cartilage". J Nutr. 123 (2 Suppl): 301–9. PMID 8429379. Unknown parameter
|month=ignored (help) - ↑ Johnson, Clarke (1998). "Biology of the Human Dentition".
- ↑ Cate, A.R. Ten. (1998). Oral Histology: development, structure, and function (5th ed.). pp. 150–5. ISBN 0-8151-2952-1.
- ↑ Taves DR, Reedy RC (1969). "A structural basis for the transphosphorylation of nucleotides with hydroxyapatite". Calcified Tissue International. 3 (1): 284–92. doi:10.1007/BF02058670. Unknown parameter
|month=ignored (help) - ↑ 44.0 44.1 Hiatt EE, Budd DA (2001). "Sedimentary phosphate formation in warm shallow waters: new insights into the palaeoceanography of the Permian Phosphoria Sea from analysis of phosphate oxygen isotopes". Sedimentary Geol. 145 (1–2): 119–33. doi:10.1016/S0037-0738(01)00127-0. Unknown parameter
|month=ignored (help)