Endoplasmic reticulum
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
The endoplasmic reticulum or ER is an organelle found in all eukaryotic cells that is an interconnected network of tubules, vesicles and cisternae. The lacey membranes of the endoplasmic reticulum were first seen by Keith R. Porter, Albert Claude, and Ernest F. Fullam in 1945.[1] These structures are responsible for several specialized functions: Protein translation, folding, and transport of proteins to be used in the cell membrane (e.g., transmembrane receptors and other integral membrane proteins), or to be secreted (exocytosed) from the cell (e.g., digestive enzymes); sequestration of calcium; and production and storage of glycogen, steroids, and other macromolecules.[2] The endoplasmic reticulum is part of the endomembrane system. The basic structure and composition of the ER membrane is similar to the plasma membrane.
Structure
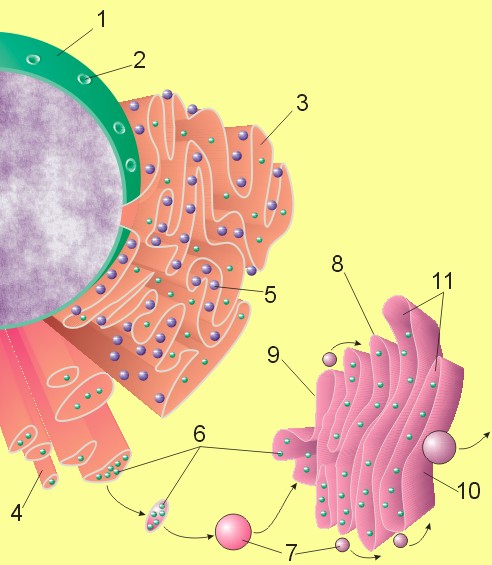
The general structure of the endoplasmic reticulum is an extensive membrane network of cisternae (sac-like structures) held together by the cytoskeleton. The phospholipid membrane encloses a space, the cisternal space (or lumen), from the cytosol. The functions of the endoplasmic reticulum vary greatly depending on the exact type of endoplasmic reticulum and the type of cell in which it resides. The three varieties are called rough endoplasmic reticulum, smooth endoplasmic reticulum, and sarcoplasmic reticulum.
Rough endoplasmic reticulum
The surface of the rough endoplasmic reticulum is studded with protein-manufacturing ribosomes giving it a "rough" appearance (hence its name).[3] But it should be noted that these ribosomes are not resident of the endoplasmic reticulum initially. The ribosomes only bind to the ER once it begins to synthesize a protein destined for sorting.[4] The free ribosome begins producing the polypeptide until a cytosolic signal recognition particle recognizes the pre-piece of 5-15 hydrophobic AAs preceded by a positively charged (basic) amino acid. This makes it easy for the complex to loop the sequence through the hydrophobic membrane. The pre-piece is then cleaved off.
The membrane of the rough endoplasmic reticulum is continuous with the outer layer of the nuclear envelope. Although there is no continuous membrane between the rough ER and the Golgi apparatus, membrane bound vesicles shuttle proteins between these two compartments.[5] COP II brings vesicles to the golgi and COP I brings the membrane back. The rough endoplasmic reticulum works in concert with the Golgi complex to target new proteins to their proper destinations.
The RER is key in producing
- lysosomal enzymes with a Mannose-6-phosphate marker added in the cis-Golgi network
- Secreted proteins, either secreted constitutively with no tag, or regulated secretion involving clathrin and paired basic amino acids in the signal peptide.
- integral membrane proteins that stay imbedded in the membrane as vesicles exit and bind to new membranes. Rab proteins are key in targeting the membrane, SNAP and SNARE proteins are key in the fusion event.
- initial glycosylation as assembly continues. This is either N-linked or O-linked (O-linked may likely occur in the golgi).
Smooth endoplasmic reticulum
The smooth endoplasmic reticulum has functions in several metabolic processes, including synthesis of lipids, metabolism of carbohydrates and calcium concentration, drug detoxification, and attachment of receptors on cell membrane proteins. It is connected to the nuclear envelope. Smooth endoplasmic reticulum is found in a variety of cell types (both animal and plant) and it serves different functions in each. The Smooth ER also contains the enzyme Glucose-6-phosphatase which converts glucose-6-phosphate to glucose, a step in gluconeogenesis. The Smooth ER consists of tubules and vesicles that branch forming a network. In some cells there are dilated areas like the sacs of rough endoplasmic reticulum. The network of smooth endoplasmic reticulum allows increased surface area for the action or storage of key enzymes and the products of these enzymes. The smooth endoplasmic reticulum is known for its storage of calcium ions in muscle cells.
Sarcoplasmic reticulum
The sarcoplasmic reticulum is a special type of smooth ER found in smooth and striated muscle. The only structural difference between this organelle and the smooth endoplasmic reticulum is the medley of protein they have, both bound to their membranes and drifting within the confines of their lumens. This fundamental difference is indicative of their functions: the smooth ER synthesizes molecules and the sarcoplasmic reticulum stores and pumps calcium ions. The sarcoplasmic reticulum contains large stores of calcium, which it sequesters and then releases when the cell is depolarized.[6] This has the effect of triggering muscle contraction.
Functions
The endoplasmic reticulum serves many general functions, including the facilitation of protein folding and the transport of synthesized proteins in sacs called cisternae.
Correct folding of newly-made proteins is made possible by several endoplasmic reticulum chaperone proteins, including protein disulfide isomerase (PDI), ERp29, the Hsp70 family member Grp78, calnexin, calreticulin, and the peptidylpropyl isomerase family. Only properly-folded proteins are transported from the rough ER to the Golgi complex.
Transport of proteins
Secretory proteins, mostly glycoproteins, are moved across the endoplasmic reticulum membrane. Proteins that are transported by the endoplasmic reticulum and from there throughout the cell are marked with an address tag called a signal sequence. The N-terminus (one end) of a polypeptide chain (i.e., a protein) contains a few amino acids that work as an address tag, which are removed when the polypeptide reaches its destination. Proteins that are destined for places outside the endoplasmic reticulum are packed into transport vesicles and moved along the cytoskeleton toward their destination.
The endoplasmic reticulum is also part of a protein sorting pathway. It is, in essence, the transportation system of the eukaryotic cell. The majority of endoplasmic reticulum resident proteins are retained in the endoplasmic reticulum through a retention motif. This motif is composed of four amino acids at the end of the protein sequence. The most common retention sequence is KDEL (lys-asp-glu-leu). However, variation on KDEL does occur and other sequences can also give rise to endoplasmic reticulum retention. It is not known if such variation can lead to sub-endoplasmic reticulum localizations. There are three KDEL receptors in mammalian cells, and they have a very high degree of sequence identity. The functional differences between these receptors remain to be established.
Other functions
- Insertion of proteins into the endoplasmic reticulum membrane: Integral proteins must be inserted into the endoplasmic reticulum membrane after they are synthesized. Insertion into the endoplasmic reticulum membrane requires the correct topogenic sequences.
- Glycosylation: Glycosylation involves the attachment of oligosaccharides.
- Disulfide bond formation and rearrangement: Disulfide bonds stabilize the tertiary and quaternary structure of many proteins.
- Drug Detoxification: The smooth ER is the site at which some drugs are detoxified.
See also
References
- ↑ Porter KR, Claude A, Fullam EF (1945 Mar). "A study of tissue culture cells by electron microscopy". J Exp Med. 81: 233–246. Check date values in:
|date=(help) - ↑ Spurger, L. (2002). Endoplasmic reticulum: Structure and function. University of Texas Medical Branch. Retrieved September 13, 2006, from http://cellbio.utmb.edu/cellbio/rer1.htm
- ↑ Campbell, Neil A. (1996) Biology Fourth Edition. Benjamin/Cummings Publishing, pp. 120-121 ISBN 0-8053-1940-9
- ↑ Lodish, Harvey, et al. (2003) Molecular Cell Biology 5th Edition. W. H. Freeman, pp. 659-666 ISBN 0716743663
- ↑ Endoplasmic reticulum. (n.d.). McGraw-Hill Encyclopedia of Science and Technology. Retrieved September 13, 2006, from Answers.com Web site: http://www.answers.com/topic/endoplasmic-reticulum
- ↑ Toyoshima C, Nakasako M, Nomura H, Ogawa H (2000). "Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution". Nature. 405 (6787): 647–55. PMID 10864315.
External links
- UMich Orientation of Proteins in Membranes localization/localization-Endoplasmic%20reticulum%20membrane
- Animations of the various cell functions referenced here
ar:شبكة إندوبلازمية bn:এন্ডোপ্লাজমিক রেটিকুলাম bg:Ендоплазмен ретикулум ca:Reticle endoplasmàtic cs:Endoplazmatické retikulum cy:Reticwlwm endoplasmig da:Endoplasmatisk reticulum de:Endoplasmatisches Retikulum gl:Retículo endoplasmático ko:소포체 hr:Endoplazmatski retikulum is:Frymisnet it:Reticolo endoplasmatico he:רשתית תוך-פלזמית lv:Endoplazmatiskais tīkls lb:Endoplasmatescht Retikulum lt:Endoplazminis tinklas mk:Ендоплазматичен ретикулум ms:Jalinan endoplasma nl:Endoplasmatisch reticulum oc:Reticulum endoplasmic sk:Endoplazmatické retikulum sl:Endoplazemski retikulum sr:Храпави ендоплазматични ретикулум fi:Endoplasmakalvosto sv:Endoplasmatiska nätverket th:เอนโดพลาสมิกเรติคูลัม