Quaternary ammonium cation
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
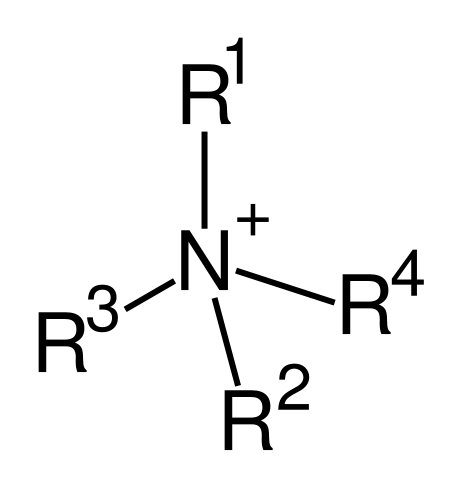
Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR4+ with R being alkyl groups. Unlike the ammonium ion NH4+ itself and primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium cations are synthesized by complete alkylation of ammonia or other amines. For possible synthesis route, see amines.
Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations with an anion. They are used as disinfectants, surfactants, fabric softeners, and as antistatic agents (e.g. in shampoo). In liquid fabric softeners, the chloride salts are often used. In dryer anticling strips, the sulfate salts are often used. This is also a common ingredient in many spermicidal jellies.
In organic chemistry, quaternary ammonium salts are used as phase transfer catalysts for reactions involving immiscible solvent systems, such as the synthesis of dichlorocarbenes with chloroform and sodium hydroxide.
The synthesis of this cation from ammonia is referred to as quaternization.
See also
- Choline
- Carnitine
- Benzalkonium chloride, a common quaternary disinfectant
- Denatonium, the most bitter compound known
- Cocamidopropyl betaine, a common surfactant
- Cetyl trimethylammonium bromide (CTAB), one of the most investigated cationic surfactant compounds
- Tetra-n-butylammonium bromide and Aliquat 336, common phase transfer catalysts
- Polyquaternium, designations for quaternary ammonium-containing polymers used for personal care products
Template:Organic-compound-stub
de:Quartäre Ammoniumverbindungen hu:Kvaterner ammóniumvegyület