Levodopa
For patient information, click here
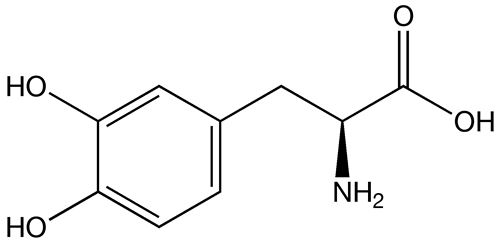 | |
| File:Levodopa3D.PNG | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Metabolism | Aromatic-L-amino-acid decarboxylase |
| Elimination half-life | 0.75–1.5 hours |
| Excretion | renal 70–80% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C9H11NO4 |
| Molar mass | 197.19 g/mol |
|
WikiDoc Resources for Levodopa |
|
Articles |
|---|
|
Most recent articles on Levodopa |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Levodopa at Clinical Trials.gov Clinical Trials on Levodopa at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Levodopa
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Levodopa Risk calculators and risk factors for Levodopa
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Levodopa |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Levodopa (INN) or L-DOPA (3,4-dihydroxy-L-phenylalanine) is an intermediate in dopamine biosynthesis. In clinical use, levodopa is administered in the management of Parkinson's disease.
Therapeutic use
Levodopa is used as a prodrug to increase dopamine levels for the treatment of Parkinson's disease, since it is able to cross the blood-brain barrier, whereas dopamine itself cannot. Once levodopa has entered the central nervous system (CNS), it is metabolized to dopamine by aromatic L-amino acid decarboxylase. However, conversion to dopamine also occurs in the peripheral tissues, causing adverse effects and decreasing the available dopamine to the CNS, so it is standard practice to co-administer a peripheral DOPA decarboxylase inhibitor – carbidopa or benserazide – and often a catechol-O-methyl transferase (COMT) inhibitor. However, Vitamin-B6 (pyridoxine) inhibits the conversion of levodopa to dopamine. Thus, it is necessary to limit pyridoxine intake, but with extreme care in dosing, for vitamin-B6 deficiency can lead to paresthesias, numbness of extremities, mental confusion, and depression.
Adverse effects
Possible adverse drug reactions include:
- Hypotension, especially if the dosage is too high
- Arrhythmias, although these are uncommon
- Nausea, which is often helped by taking the drug with food, although protein interferes with drug absorption
- Gastrointestinal bleeding
- Disturbed respiration, which is not always harmful, and can actually benefit patients with upper airway obstruction
- Hair loss
- Confusion
- Extreme emotional states, particularly anxiety, but also excessive libido
- Vivid dreams and/or fragmented sleep
- Visual and possibly auditory hallucinations
- Effects on learning; there is some evidence that it improves working memory, while impairing other complex functions
- Sleepiness and sleep attacks
- A condition similar to amphetamine psychosis.
Although there are many adverse effects associated with levodopa, particularly psychiatric ones, it has fewer than other anti-Parkinson's drugs, including anticholinergics, amantadine, and dopamine agonists.
More serious are the effects of chronic levodopa administration, which include:
- End-of-dose deterioration of function
- On/off oscillations
- Freezing during movement
- Dose failure (drug resistance)
- Dyskinesia at peak dose.
Clinicians will try to avoid these by limiting levodopa dosages as far as possible until absolutely necessary.
Biosynthesis
L-DOPA is produced from the amino acid tyrosine by the enzyme tyrosine hydroxylase. It is also the precursor molecule for the catecholamine neurotransmitters dopamine and norepinephrine (noradrenaline), and the hormone epinephrine (adrenaline). Dopamine is formed by the decarboxylation of L-DOPA.
The prefix L- references its property of levorotation (compared with dextrorotation or D-DOPA).
History
In work that earned him a Nobel Prize in 2000, Swedish scientist Arvid Carlsson first showed in the 1950s that administering levodopa to animals with Parkinsonian symptoms would cause a reduction of the symptoms. The neurologist Oliver Sacks describes this treatment in human patients with encephalitis lethargica in his book Awakenings, upon which the movie Awakenings is based.
The 2001 Nobel Prize in Chemistry was also related to L-DOPA: the Nobel Committee awarded one-fourth of the prize to William S. Knowles for his work on chirally-catalysed hydrogenation reactions, the most noted example of which was used for the synthesis of L-DOPA.
Supplements containing L-DOPA
Herbal supplements containing standardized dosages of L-DOPA are available without a prescription. These supplements have recently increased in both availability and popularity in the United States and on the Internet. The most common plant source of L-DOPA marketed in this manner is a tropical legume, Mucuna pruriens, also known as "Velvet Bean" and by a number of other common names.
Two of the most popular brands of Mucuna pruriens are "DopaBean," marketed by Solaray, and "Mucuna," marketed by Physician Formulas, Inc. These preparations claim to contain standardized dosages of L-DOPA in enteric-coated capsules. The dosage claimed is usually about 50 mg per capsule, and the recommended dose is two capsules per day. A third product, "L-Dopa," marketed by Unique Nutrition, claims a higher effective dose of 250 mg. American Nutrition also carries a Mucuna pruriens standardized to 40% L-DOPA under its NutraceuticsRx label.
Some of the claims made for the use of these supplements may have validity, whereas many do not. Among the most common claims are that the supplements will increase libido and aid in body-building (presumably by increasing human growth hormone in both cases). The long-term consequences of the use of these supplements by healthy individuals remains to be seen.
Adhesion
DOPA is a key molecule in the formation of marine adhesive proteins, such as those found in mussels. It is believed to be responsible for the water-resistance and rapid curing abilities of these proteins. DOPA may also be used to prevent surfaces from fouling by bonding antifouling polymers to a susceptible substrate.
Melanin formation
Both levodopa and the related amino acid L-tyrosine are precursors to the biological pigment melanin. The enzyme tyrosinase catalyzes the oxidation of L-dopa to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers.
References
- Waite, J. Herbert; et al. (2005). "Mussel Adhesion: Finding the Tricks Worth Mimicking". J Adhesion. 81: 1–21.
- Messersmith, Phillip B.; et al. (2006). "Rapid Gel Formation and Adhesion in Photocurable and Biodegradable Block Copolymers with High DOPA Content". Macromolecules. 39: 1740–1748.
de:Levodopa et:Levodopa it:L-DOPA nl:Levodopa fi:L-dopa sv:Levodopa
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- CS1 maint: Explicit use of et al.
- Dopamine agonists
- Psychoactive drugs
- Amino acids
- Prodrugs
- Drugs
- Neurology
- Overview complete