Eplerenone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Eplerenone is a aldosterone antagonist that is FDA approved for the {{{indicationType}}} of hypertension and congestive heart failure after myocardial infarction. Common adverse reactions include hyperkalemia, diarrhea, dizziness, elevated serum creatinine, cough, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Congestive Heart Failure Post-Myocardial Infarction
Eplerenone tablets are indicated to improve survival of stable patients with left ventricular (LV) systolic dysfunction (ejection fraction ≤40%) and clinical evidence of congestive heart failure (CHF) after an acute myocardial infarction.
- Dosing Information
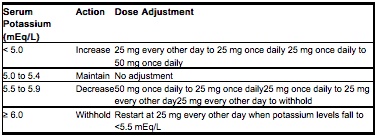
- Treatment should be initiated at 25 mg once daily and titrated to the recommended dose of 50 mg once daily, preferably within 4 weeks as tolerated by the patient.
- Eplerenone tablets may be administered with or without food.
- Once treatment with eplerenone tablets have begun, adjust the dose based on the serum potassium level as shown in the table below.
Hypertension
- Dosing Information
- The recommended starting dose of eplerenone tablets are 50 mg once daily.
- The full therapeutic effect of eplerenone tablets is apparent within 4 weeks.
- For patients with an inadequate blood pressure response to 50 mg once daily the dosage of eplerenone tablets should be increased to 50 mg twice daily. Higher dosages of eplerenone tablets are not recommended because they have no greater effect on blood pressure than 100 mg and are associated with an increased risk of hyperkalemia.
Dose Modifications for Specific Populations
- Serum potassium levels should be measured before initiating eplerenone tablet therapy, and eplerenone tablets should not be prescribed if serum potassium is >5.5 mEq/L.
- For hypertensive patients receiving moderate CYP3A4 inhibitors (e.g., erythromycin, saquinavir, verapamil, and fluconazole), the starting dose of eplerenone tablets should be reduced to 25 mg once daily.
- No adjustment of the starting dose is recommended for the elderly or for patients with mild-to-moderate hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Heart Failure, Myocardial infarction with Complication
- Developed by: American College of Cardiology (ACC) and American Heart Association (AHA)
- Class of Recommendation: Class IIa
- Strength of Evidence: Category A
- Dosing Information
- 25 mg daily initially, titrated to a maximum of 50 mg/day[1]
Non–Guideline-Supported Use
Albuminuria in Diabetes Mellitus
- Dosing Information
- 50 mg once daily[2]
Low-Renin Essential Hypertension
- Dosing Information
- 100 mg once daily[3]
Systolic Heart Failure (Mild)
- Dosing Information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Eplerenone tablets has not been studied in hypertensive patients less than 4 years old because the study in older pediatric patients did not demonstrate effectiveness.
- Eplerenone has not been studied in pediatric patients with heart failure.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eplerenone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eplerenone in pediatric patients.
Contraindications
For All Patients
- Serum potassium >5.5 mEq/L at initiation
- Creatinine clearance ≤30 mL/min
- Concomitant administration of strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, and nelfinavir)
For Patients Treated for Hypertension
- Type 2 diabetes with microalbuminuria
- Serum creatinine >2.0 mg/dL in males or >1.8 mg/dL in females
- Creatinine clearance <50 mL/min
- Concomitant administration of potassium supplements or potassium-sparing diuretics (e.g., amiloride, spironolactone, or triamterene)
Warnings
Hyperkalemia
- Minimize the risk of hyperkalemia with proper patient selection and monitoring, and avoidance of certain concomitant medications. Monitor patients for the development of hyperkalemia until the effect of eplerenone tablets are established. Patients who develop hyperkalemia (>5.5 mEq/L) may continue eplerenone tablets therapy with proper dose adjustment. Dose reduction decreases potassium levels.
- The rates of hyperkalemia increase with declining renal function. Patients with hypertension who have serum creatinine levels >2.0 mg/dL (males) or >1.8 mg/dL (females) or creatinine clearance ≤50 mL/min should not be treated with eplerenone tablets. Patients with CHF post-MI who have serum creatinine levels >2.0 mg/dL (males) or >1.8 mg/dL (females) or creatinine clearance ≤50 mL/min should be treated with eplerenone tablets with caution.
- Diabetic patients with CHF post-MI should also be treated with caution, especially those with proteinuria. The subset of patients in the EPHESUS study with both diabetes and proteinuria on the baseline urinalysis had increased rates of hyperkalemia compared to patients with either diabetes or proteinuria.
- The risk of hyperkalemia may increase when eplerenone is used in combination with an angiotensin converting enzyme (ACE) inhibitor and/or an angiotensin receptor blocker (ARB).
Impaired Hepatic Function
- Mild-to-moderate hepatic impairment did not increase the incidence of hyperkalemia. In 16 subjects with mild-to-moderate hepatic impairment who received 400 mg of eplerenone, no elevations of serum potassium above 5.5 mEq/L were observed. The mean increase in serum potassium was 0.12 mEq/L in patients with hepatic impairment and 0.13 mEq/L in normal controls. The use of eplerenone tablets in patients with severe hepatic impairment has not been evaluated.
Impaired Renal Function
- Patients with decreased renal function are at increased risk of hyperkalemia.
Adverse Reactions
Clinical Trials Experience
Congestive Heart Failure Post-Myocardial Infarction
- In EPHESUS, safety was evaluated in 3307 patients treated with eplerenone tablets and 3301 placebo-treated patients. The overall incidence of adverse events reported with eplerenone tablets (78.9%) was similar to placebo (79.5%). Adverse events occurred at a similar rate regardless of age, gender, or race. Patients discontinued treatment due to an adverse event at similar rates in either treatment group (4.4% eplerenone tablets vs. 4.3% placebo), with the most common reasons for discontinuation being hyperkalemia, myocardial infarction, and abnormal renal function.
- Adverse reactions that occurred more frequently in patients treated with eplerenone tablets than placebo were hyperkalemia (3.4% vs. 2.0%) and increased creatinine (2.4% vs. 1.5%). Discontinuations due to hyperkalemia or abnormal renal function were less than 1.0% in both groups. Hypokalemia occurred less frequently in patients treated with eplerenone tablets (0.6% vs. 1.6%).
- The rates of sex hormone-related adverse events are shown in the table below.

Hypertension
- Eplerenone tablets has been evaluated for safety in 3091 patients treated for hypertension. A total of 690 patients were treated for over 6 months and 106 patients were treated for over 1 year.
- In placebo-controlled studies, the overall rates of adverse events were 47% with eplerenone tablets and 45% with placebo. Adverse events occurred at a similar rate regardless of age, gender, or race. Therapy was discontinued due to an adverse event in 3% of patients treated with eplerenone tablets and 3% of patients given placebo. The most common reasons for discontinuation of eplerenone tablets were headache, dizziness, angina pectoris/myocardial infarction, and increased GGT. The adverse events that were reported at a rate of at least 1% of patients and at a higher rate in patients treated with eplerenone tablets in daily doses of 25 to 400 mg versus placebo are shown in the table below.

- Gynecomastia and abnormal vaginal bleeding were reported with eplerenone tablets but not with placebo. The rates of these sex hormone-related adverse events are shown in the table below. The rates increased slightly with increasing duration of therapy. In females, abnormal vaginal bleeding was also reported in 0.8% of patients on antihypertensive medications (other than spironolactone) in active control arms of the studies with eplerenone tablets.

Clinical Laboratory Test Findings
Congestive Heart Failure Post-Myocardial Infarction
- Increases of more than 0.5 mg/dL were reported for 6.5% of patients administered eplerenone tablets and for 4.9% of placebo-treated patients.
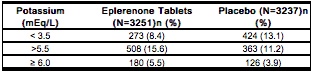
- In EPHESUS, the frequencies of patients with changes in potassium (<3.5 mEq/L or >5.5 mEq/L or ≥6.0 mEq/L) receiving eplerenone tablets compared with placebo are displayed in the table below.
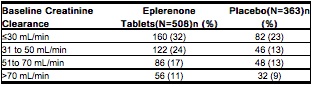
- The table below shows the rates of hyperkalemia in EPHESUS as assessed by baseline renal function (creatinine clearance).
- The table below shows the rates of hyperkalemia in EPHESUS as assessed by two baseline characteristics: presence/absence of proteinuria from baseline urinalysis and presence/absence of diabetes.

Hypertension

- Patients with both type 2 diabetes and microalbuminuria are at increased risk of developing persistent hyperkalemia. In a study of such patients taking eplerenone tablets 200 mg, the frequencies of maximum serum potassium levels >5.5 mEq/L were 33% with eplerenone tablets given alone and 38% when eplerenone tablets were given with enalapril.
- Rates of hyperkalemia increased with decreasing renal function. In all studies, serum potassium elevations >5.5 mEq/L were observed in 10.4% of patients treated with eplerenone tablets with baseline calculated creatinine clearance <70 mL/min, 5.6% of patients with baseline creatinine clearance of 70 to 100 mL/min, and 2.6% of patients with baseline creatinine clearance of >100 mL/min.
- Serum triglycerides increased in a dose-related manner. Mean increases ranged from 7.1 mg/dL at 50 mg daily to 26.6 mg/dL at 400 mg daily. Increases in triglycerides (above 252 mg/dL) were reported for 15% of patients administered eplerenone tablets and 12% of placebo-treated patients.
- Serum cholesterol increased in a dose-related manner. Mean changes ranged from a decrease of 0.4 mg/dL at 50 mg daily to an increase of 11.6 mg/dL at 400 mg daily. Increases in serum cholesterol values greater than 200 mg/dL were reported for 0.3% of patients administered eplerenone tablets and 0% of placebo-treated patients.
- Liver Function Tests
- Serum alanine aminotransferase (ALT) and gamma glutamyl transpeptidase (GGT) increased in a dose-related manner. Mean increases ranged from 0.8 U/L at 50 mg daily to 4.8 U/L at 400 mg daily for ALT and 3.1 U/L at 50 mg daily to 11.3 U/L at 400 mg daily for GGT. Increases in ALT levels greater than 120 U/L (3 times upper limit of normal) were reported for 15/2259 patients administered eplerenone tablets and 1/351 placebo-treated patients. Increases in ALT levels greater than 200 U/L (5 times upper limit of normal) were reported for 5/2259 of patients administered eplerenone tablets and 1/351 placebo-treated patients. Increases of ALT greater than 120 U/L and bilirubin greater than 1.2 mg/dL were reported 1/2259 patients administered eplerenone tablets and 0/351 placebo-treated patients. Hepatic failure was not reported in patients receiving eplerenone tablets.
- Serum creatinine increased in a dose-related manner. Mean increases ranged from 0.01 mg/dL at 50 mg daily to 0.03 mg/dL at 400 mg daily. Increases in blood urea nitrogen to greater than 30 mg/dL and serum creatinine to greater than 2 mg/dL were reported for 0.5% and 0.2%, respectively, of patients administered eplerenone tablets and 0% of placebo-treated patients.
- Increases in uric acid to greater than 9 mg/dL were reported in 0.3% of patients administered eplerenone tablets and 0% of placebo-treated patients.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of eplerenone tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin
Drug Interactions
- CYP3A4 Inhibitors
- The risk of hyperkalemia may increase when eplerenone is used in combination with an angiotensin converting enzyme (ACE) inhibitor and/or an angiotensin receptor blocker (ARB). A close monitoring of serum potassium and renal function is recommended, especially in patients at risk for impaired renal function, e.g., the elderly.
- Congestive Heart Failure Post-Myocardial Infarction
- In EPHESUS, 3020 (91%) patients receiving eplerenone tablets 25 to 50 mg also received angiotensin II receptor antagonists. Rates of patients with maximum potassium levels >5.5 mEq/L were similar regardless of the use of angiotensin II receptor antagonists.
- Hypertension
- In clinical studies of patients with hypertension, the addition of eplerenone 50 to 100 mg to angiotensin II receptor antagonists increased mean serum potassium slightly (about 0.09 to 0.13 mEq/L).
- A drug interaction study of eplerenone with lithium has not been conducted. Lithium toxicity has been reported in patients receiving lithium concomitantly with diuretics and ACE inhibitors. Serum lithium levels should be monitored frequently if eplerenone tablets is administered concomitantly with lithium.
- A drug interaction study of eplerenone with an NSAID has not been conducted. The administration of other potassium-sparing antihypertensives with NSAIDs has been shown to reduce the antihypertensive effect in some patients and result in severe hyperkalemia in patients with impaired renal function. Therefore, when eplerenone tablets and NSAIDs are used concomitantly, patients should be observed to determine whether the desired effect on blood pressure is obtained and monitored for changes in serum potassium levels.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- There are no adequate and well-controlled studies in pregnant women. Eplerenone tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Embryo-fetal development studies were conducted with doses up to 1000 mg/kg/day in rats and 300 mg/kg/day in rabbits (exposures up to 32 and 31 times the human AUC for the 100 mg/day therapeutic dose, respectively). No teratogenic effects were seen in rats or rabbits, although decreased body weight in maternal rabbits and increased rabbit fetal resorptions and post-implantation loss were observed at the highest administered dosage. Because animal reproduction studies are not always predictive of human response, eplerenone tablets should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Eplerenone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Eplerenone during labor and delivery.
Nursing Mothers
- The concentration of eplerenone in human breast milk after oral administration is unknown. However, preclinical data show that eplerenone and/or metabolites are present in rat breast milk (0.85:1 [milk: plasma] AUC ratio) obtained after a single oral dose. Peak concentrations in plasma and milk were obtained from 0.5 to 1 hour after dosing. Rat pups exposed by this route developed normally. Because many drugs are excreted in human milk and because of the unknown potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- In a 10-week study of 304 hypertensive pediatric patients age 4 to 17 years treated with eplerenone tablets up to 100 mg per day, doses that produced exposure similar to that in adults, eplerenone tablets did not lower blood pressure effectively. In this study and in a 1-year pediatric safety study in 149 patients, the incidence of reported adverse events was similar to that of adults.
- Eplerenone tablets has not been studied in hypertensive patients less than 4 years old because the study in older pediatric patients did not demonstrate effectiveness.
- Eplerenone has not been studied in pediatric patients with heart failure.
Geriatic Use
- Congestive Heart Failure Post-Myocardial Infarction
- Of the total number of patients in EPHESUS, 3340 (50%) were 65 and over, while 1326 (20%) were 75 and over. Patients greater than 75 years did not appear to benefit from the use of eplerenone tablets.
- No differences in overall incidence of adverse events were observed between elderly and younger patients. However, due to age-related decreases in creatinine clearance, the incidence of laboratory-documented hyperkalemia was increased in patients 65 and older.
- Hypertension
- Of the total number of subjects in clinical hypertension studies of eplerenone tablets, 1123 (23%) were 65 and over, while 212 (4%) were 75 and over. No overall differences in safety or effectiveness were observed between elderly subjects and younger subjects.
Gender
There is no FDA guidance on the use of Eplerenone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Eplerenone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Eplerenone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Eplerenone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Eplerenone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Eplerenone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Serum potassium should be measured before initiating eplerenone tablet therapy, within the first week, and at one month after the start of treatment or dose adjustment. Serum potassium should be assessed periodically thereafter. Patient characteristics and serum potassium levels may indicate that additional monitoring is appropriate. In the EPHESUS study, the majority of hyperkalemia was observed within the first three months after randomization.
- In all patients taking eplerenone tablets who start taking a moderate CYP3A4 inhibitor, check serum potassium and serum creatinine in 3 to 7 days.
IV Compatibility
There is limited information regarding IV Compatibility of Eplerenone in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- No cases of human overdosage with eplerenone have been reported.
- Lethality was not observed in mice, rats, or dogs after single oral doses that provided Cmax exposures at least 25 times higher than in humans receiving eplerenone 100 mg/day.
- Dogs showed emesis, salivation, and tremors at a Cmax 41 times the human therapeutic Cmax, progressing to sedation and convulsions at higher exposures.
- The most likely manifestation of human overdosage would be anticipated to be hypotension or hyperkalemia.
Management
- Eplerenone cannot be removed by hemodialysis.
- Eplerenone has been shown to bind extensively to charcoal.
- If symptomatic hypotension should occur, supportive treatment should be instituted.
- If hyperkalemia develops, standard treatment should be initiated.
Chronic Overdose
There is limited information regarding Chronic Overdose of Eplerenone in the drug label.
Pharmacology
| |
Eplerenone
| |
| Systematic (IUPAC) name | |
| pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo, γ-lactone, methyl ester (7α, 11α, 17α) | |
| Identifiers | |
| CAS number | |
| ATC code | C03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 414.49 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 69% |
| Metabolism | hepatic (CYP3A4) |
| Half life | 6-8 hours |
| Excretion | 67% renal 32% biliary |
| Therapeutic considerations | |
| Pregnancy cat. |
B3 (Aust) |
| Legal status | |
| Routes | oral |
Mechanism of Action
- Eplerenone binds to the mineralocorticoid receptor and blocks the binding of aldosterone, a component of the renin-angiotensin-aldosterone-system (RAAS). Aldosterone synthesis, which occurs primarily in the adrenal gland, is modulated by multiple factors, including angiotensin II and non-RAAS mediators such as adrenocorticotropic hormone (ACTH) and potassium. Aldosterone binds to mineralocorticoid receptors in both epithelial (e.g., kidney) and nonepithelial (e.g., heart, blood vessels, and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms.
- Eplerenone has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone.
- Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone, and androgen receptors.
Structure
- Eplerenone tablets contain eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor.
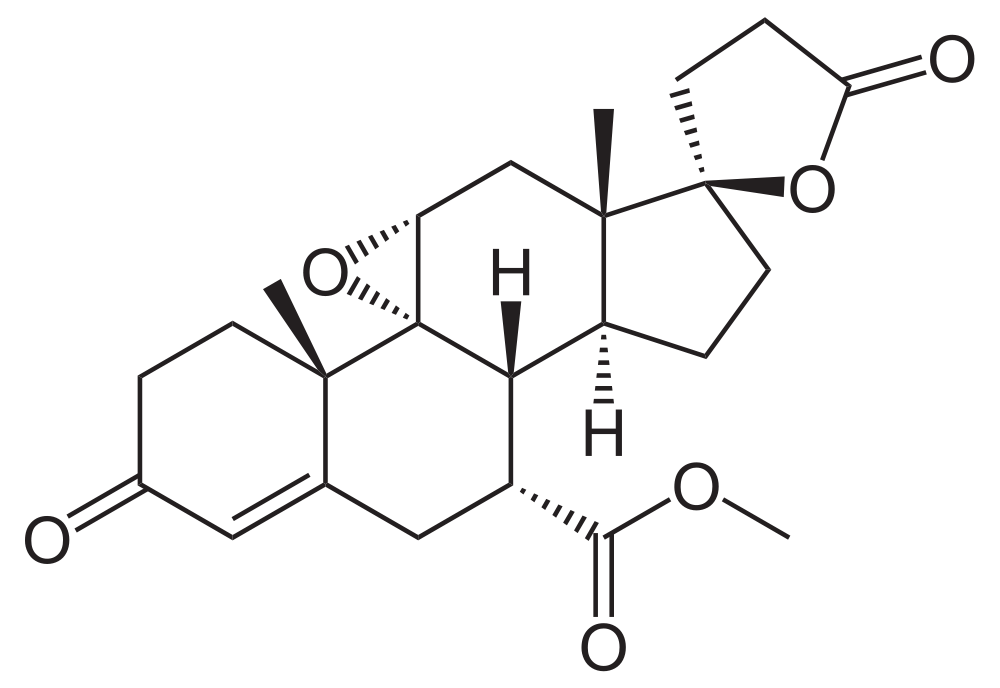
- Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, γ-lactone, methyl ester, (7α,11α,17α)-. Its empirical formula is C24H30O6 and it has a molecular weight of 414.50. The structural formula of eplerenone is represented below:
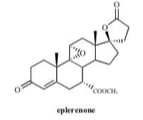
- Eplerenone is an odorless, white to off-white crystalline powder. It is very slightly soluble in water, with its solubility essentially pH-independent. The octanol/water partition coefficient of eplerenone is approximately 7.1 at pH 7.0.
- Eplerenone tablets for oral administration contains 25 mg or 50 mg of eplerenone and the following inactive ingredients: microcrystalline cellulose, povidone, croscarmellose sodium, hypromellose, hydroxypropyl cellulose, magnesium stearate, titanium dioxide, polyethylene glycol, and iron oxide yellow.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Eplerenone in the drug label.
Pharmacokinetics
- Eplerenone is cleared predominantly by cytochrome P450 (CYP) 3A4 metabolism, with an elimination half-life of 4 to 6 hours. Steady state is reached within 2 days. Absorption is not affected by food. Inhibitors of CYP3A4 (e.g., ketoconazole, saquinavir) increase blood levels of eplerenone.
Absorption and Distribution
- Mean peak plasma concentrations of eplerenone are reached approximately 1.5 hours following oral administration. The absolute bioavailability of eplerenone is 69% following administration of a 100 mg oral tablet. Both peak plasma levels (Cmax) and area under the curve (AUC) are dose proportional for doses of 25 to 100 mg and less than proportional at doses above 100 mg.
- The plasma protein binding of eplerenone is about 50% and it is primarily bound to alpha 1-acid glycoproteins. The apparent volume of distribution at steady state ranged from 43 to 90 L. Eplerenone does not preferentially bind to red blood cells.
Metabolism and Excretion
- Eplerenone metabolism is primarily mediated via CYP3A4. No active metabolites of eplerenone have been identified in human plasma.
- Less than 5% of an eplerenone dose is recovered as unchanged drug in the urine and feces. Following a single oral dose of radiolabeled drug, approximately 32% of the dose was excreted in the feces and approximately 67% was excreted in the urine. The elimination half-life of eplerenone is approximately 4 to 6 hours. The apparent plasma clearance is approximately 10 L/hr.
Age, Gender, and Race
- The pharmacokinetics of eplerenone at a dose of 100 mg once daily has been investigated in the elderly (≥65 years), in males and females, and in Blacks. At steady state, elderly subjects had increases in Cmax (22%) and AUC (45%) compared with younger subjects (18 to 45 years). The pharmacokinetics of eplerenone did not differ significantly between males and females. At steady state, Cmax was 19% lower and AUC was 26% lower in Blacks.
Renal Impairment
- The pharmacokinetics of eplerenone was evaluated in patients with varying degrees of renal impairment and in patients undergoing hemodialysis. Compared with control subjects, steady state AUC and Cmax were increased by 38% and 24%, respectively, in patients with severe renal impairment and were decreased by 26% and 3%, respectively, in patients undergoing hemodialysis. No correlation was observed between plasma clearance of eplerenone and creatinine clearance. Eplerenone is not removed by hemodialysis.
Hepatic Impairment
- The pharmacokinetics of eplerenone 400 mg has been investigated in patients with moderate (Child-Pugh Class B) hepatic impairment and compared with normal subjects. Steady state Cmax and AUC of eplerenone were increased by 3.6% and 42%, respectively.
Heart Failure
- The pharmacokinetics of eplerenone 50 mg was evaluated in 8 patients with heart failure (NYHA classification II–IV) and 8 matched (gender, age, weight) healthy controls. Compared with the controls, steady state AUC and Cmax in patients with stable heart failure were 38% and 30% higher, respectively.
Drug-Drug Interactions
- Eplerenone is metabolized primarily by CYP3A4. Inhibitors of CYP3A4 cause increased exposure.
- Drug-drug interaction studies were conducted with a 100 mg dose of eplerenone.
- A pharmacokinetic study evaluating the administration of a single dose of eplerenone tablets 100 mg with ketoconazole 200 mg two times a day, a strong inhibitor of the CYP3A4 pathway, showed a 1.7-fold increase in Cmax of eplerenone and a 5.4-fold increase in AUC of eplerenone.
- Administration of eplerenone with moderate CYP3A4 inhibitors (e.g., erythromycin 500 mg BID, verapamil 240 mg once daily, saquinavir 1200 mg three times a day, fluconazole 200 mg once daily) resulted in increases in Cmax of eplerenone ranging from 1.4- to 1.6-fold and AUC from 2.0- to 2.9-fold.
- Grapefruit juice caused only a small increase (about 25%) in exposure.
- Eplerenone is not an inhibitor of CYP1A2, CYP3A4, CYP2C19, CYP2C9, or CYP2D6. Eplerenone did not inhibit the metabolism of amiodarone, amlodipine, astemizole, chlorzoxazone, cisapride, dexamethasone, dextromethorphan, diclofenac, 17α-ethinyl estradiol, fluoxetine, losartan, lovastatin, mephobarbital, methylphenidate, methylprednisolone, metoprolol, midazolam, nifedipine, phenacetin, phenytoin, simvastatin, tolbutamide, triazolam, verapamil, and warfarin in vitro. Eplerenone is not a substrate or an inhibitor of P-glycoprotein at clinically relevant doses.
- No clinically significant drug-drug pharmacokinetic interactions were observed when eplerenone was administered with cisapride, cyclosporine, digoxin, glyburide, midazolam, oral contraceptives (norethindrone/ethinyl estradiol), simvastatin, or warfarin. St. John's wort (a CYP3A4 inducer) caused a small (about 30%) decrease in eplerenone AUC.
- No significant changes in eplerenone pharmacokinetics were observed when eplerenone was administered with aluminum- and magnesium-containing antacids.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Eplerenone was non-genotoxic in a battery of assays including in vitro bacterial mutagenesis (Ames test in Salmonella spp. and E. coli), in vitro mammalian cell mutagenesis (mouse lymphoma cells), in vitro chromosomal aberration (Chinese hamster ovary cells), in vivo rat bone marrow micronucleus formation, and in vivo/ex vivo unscheduled DNA synthesis in rat liver.
- There was no drug-related tumor response in heterozygous p53 deficient mice when tested for 6 months at dosages up to 1000 mg/kg/day (systemic AUC exposures up to 9 times the exposure in humans receiving the 100 mg/day therapeutic dose). Statistically significant increases in benign thyroid tumors were observed after 2 years in both male and female rats when administered eplerenone 250 mg/kg/day (highest dose tested) and in male rats only at 75 mg/kg/day. These dosages provided systemic AUC exposures approximately 2 to 12 times higher than the average human therapeutic exposure at 100 mg/day. Repeat dose administration of eplerenone to rats increases the hepatic conjugation and clearance of thyroxin, which results in increased levels of TSH by a compensatory mechanism. Drugs that have produced thyroid tumors by this rodent-specific mechanism have not shown a similar effect in humans.
- Male rats treated with eplerenone at 1000 mg/kg/day for 10 weeks (AUC 17 times that at the 100 mg/day human therapeutic dose) had decreased weights of seminal vesicles and epididymides and slightly decreased fertility. Dogs administered eplerenone at dosages of 15 mg/kg/day and higher (AUC 5 times that at the 100 mg/day human therapeutic dose) had dose-related prostate atrophy. The prostate atrophy was reversible after daily treatment for 1 year at 100 mg/kg/day. Dogs with prostate atrophy showed no decline in libido, sexual performance, or semen quality. Testicular weight and histology were not affected by eplerenone in any test animal species at any dosage.
Clinical Studies
Congestive Heart Failure Post-Myocardial Infarction
- The eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) was a multinational, multicenter, double-blind, randomized, placebo-controlled study in patients clinically stable 3 to 14 days after an acute myocardial infarction (MI) with left ventricular dysfunction (as measured by left ventricular ejection fraction [LVEF] ≤40%) and either diabetes or clinical evidence of congestive heart failure (CHF) (pulmonary congestion by exam or chest x-ray or S3). Patients with CHF of valvular or congenital etiology, patients with unstable post-infarct angina, and patients with serum potassium >5.0 mEq/L or serum creatinine >2.5 mg/dL were to be excluded. Patients were allowed to receive standard post-MI drug therapy and to undergo revascularization by angioplasty or coronary artery bypass graft surgery.
- Patients randomized to eplerenone tablets were given an initial dose of 25 mg once daily and titrated to the target dose of 50 mg once daily after 4 weeks if serum potassium was < 5.0 mEq/L. Dosage was reduced or suspended anytime during the study if serum potassium levels were ≥ 5.5 mEq/L.
- EPHESUS randomized 6,632 patients (9.3% U.S.) at 671 centers in 27 countries. The study population was primarily white (90%, with 1% Black, 1% Asian, 6% Hispanic, 2% other) and male (71%). The mean age was 64 years (range, 22 to 94 years). The majority of patients had pulmonary congestion (75%) by exam or x-ray and were Killip Class II (64%). The mean ejection fraction was 33%. The average time to enrollment was 7 days post-MI. Medical histories prior to the index MI included hypertension (60%), coronary artery disease (62%), dyslipidemia (48%), angina (41%), type 2 diabetes (30%), previous MI (27%), and CHF (15%).
- The mean dose of eplerenone tablets was 43 mg/day. Patients also received standard care including aspirin (92%), ACE inhibitors (90%), β-blockers (83%), nitrates (72%), loop diuretics (66%), or HMG-CoA reductase inhibitors (60%).
- Patients were followed for an average of 16 months (range, 0 to 33 months). The ascertainment rate for vital status was 99.7%.
- The co-primary endpoints for EPHESUS were (1) the time to death from any cause, and (2) the time to first occurrence of either cardiovascular (CV) mortality [defined as sudden cardiac death or death due to progression of congestive heart failure (CHF), stroke, or other CV causes] or CV hospitalization (defined as hospitalization for progression of CHF, ventricular arrhythmias, acute myocardial infarction, or stroke).
- For the co-primary endpoint for death from any cause, there were 478 deaths in the eplerenone tablets group (14.4%) and 554 deaths in the placebo group (16.7%). The risk of death with eplerenone tablets was reduced by 15% [hazard ratio equal to 0.85 (95% confidence interval 0.75 to 0.96; p = 0.008 by log rank test)]. Kaplan-Meier estimates of all-cause mortality are shown in the figure below and the components of mortality are provided in the table below.


- The time to first event for the co-primary endpoint of CV death or hospitalization, as defined above, was longer in the eplerenone tablets group (hazard ratio 0.87, 95% confidence interval 0.79 to 0.95, p = 0.002). An analysis that included the time to first occurrence of CV mortality and all CV hospitalizations (atrial arrhythmia, angina, CV procedures, progression of CHF, MI, stroke, ventricular arrhythmia, or other CV causes) showed a smaller effect with a hazard ratio of 0.92 (95% confidence interval 0.86 to 0.99; p = 0.028). The combined endpoints, including combined all-cause hospitalization and mortality were driven primarily by CV mortality. The combined endpoints in EPHESUS, including all-cause hospitalization and all-cause mortality, are presented in the table below.
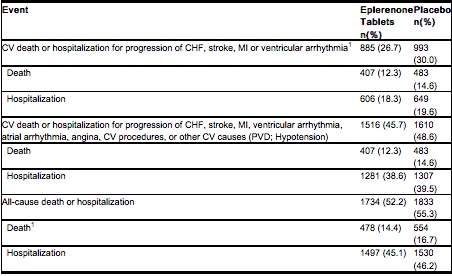
- mortality hazard ratios varied for some subgroups as shown in the figure below. mortality hazard ratios appeared favorable for eplerenone tablets for both genders and for all races or ethnic groups, although the numbers of non-Caucasians were low (648, 10%). Patients with diabetes without clinical evidence of CHF and patients greater than 75 years did not appear to benefit from the use of eplerenone tablets. Such subgroup analyses must be interpreted cautiously.

- Analyses conducted for a variety of CV biomarkers did not confirm a mechanism of action by which mortality was reduced.
Hypertension
- The safety and efficacy of eplerenone tablets have been evaluated alone and in combination with other antihypertensive agents in clinical studies of 3091 hypertensive patients. The studies included 46% women, 14% Blacks, and 22% elderly (age ≥65). The studies excluded patients with elevated baseline serum potassium (>5.0 mEq/L) and elevated baseline serum creatinine (generally >1.5 mg/dL in males and >1.3 mg/dL in females).
- Two fixed-dose, placebo-controlled, 8- to 12-week monotherapy studies in patients with baseline diastolic blood pressures of 95 to 114 mm Hg were conducted to assess the antihypertensive effect of eplerenone tablets. In these two studies, 611 patients were randomized to eplerenone tablets and 140 patients to placebo. Patients received eplerenone tablets in doses of 25 to 400 mg daily as either a single daily dose or divided into two daily doses. The mean placebo-subtracted reductions in trough cuff blood pressure achieved by eplerenone tablets in these studies at doses up to 200 mg are shown in the figures below.

- Patients treated with eplerenone tablets 50 to 200 mg daily experienced significant decreases in sitting systolic and diastolic blood pressure at trough with differences from placebo of 6 to 13 mm Hg (systolic) and 3 to 7 mm Hg (diastolic). These effects were confirmed by assessments with 24-hour ambulatory blood pressure monitoring (ABPM). In these studies, assessments of 24-hour ABPM data demonstrated that eplerenone tablets, administered once or twice daily, maintained antihypertensive efficacy over the entire dosing interval. However, at a total daily dose of 100 mg, eplerenone tablets administered as 50 mg twice per day produced greater trough cuff (4/3 mm Hg) and ABPM (2/1 mm Hg) blood pressure reductions than 100 mg given once daily.
- Blood pressure lowering was apparent within 2 weeks from the start of therapy with eplerenone, with maximal antihypertensive effects achieved within 4 weeks. Stopping eplerenone tablets following treatment for 8 to 24 weeks in six studies did not lead to adverse event rates in the week following withdrawal of eplerenone tablets greater than following placebo or active control withdrawal. Blood pressures in patients not taking other antihypertensives rose 1 week after withdrawal of eplerenone tablets by about 6/3 mm Hg, suggesting that the antihypertensive effect of eplerenone tablets was maintained through 8 to 24 weeks.
- Blood pressure reductions with eplerenone tablets in the two fixed-dose monotherapy studies and other studies using titrated doses, as well as concomitant treatments, were not significantly different when analyzed by age, gender, or race with one exception. In a study in patients with low renin hypertension, blood pressure reductions in Blacks were smaller than those in whites during the initial titration period with eplerenone tablets.
- Eplerenone tablets has been studied concomitantly with treatment with, angiotensin II receptor antagonists, calcium channel blockers, beta blockers, and hydrochlorothiazide. When administered concomitantly with one of these drugs eplerenone tablets usually produced its expected antihypertensive effects.
- There was no significant change in average heart rate among patients treated with eplerenone tablets in the combined clinical studies. No consistent effects of eplerenone tablets on heart rate, QRS duration, or PR or QT interval were observed in 147 normal subjects evaluated for electrocardiographic changes during pharmacokinetic studies.
How Supplied
- Eplerenone tablets 25 mg are available for oral administration as yellow, round, film-coated tablets, unscored and engraved “EP” over “25” on one side, and “APO” on the other side. They are supplied as follows:
- Bottles of 30 (NDC 60505-2651-3)
- Bottles of 90 (NDC 60505-2651-9)
- Bottles of 500 (NDC 60505-2651-5)
- Bottles of 1000 (NDC 60505-2651-8)
- Blisters of 100 (NDC 60505-2651-7)
- Eplerenone tablets 50 mg are available for oral administration as yellow, round, film-coated tablets, unscored and engraved “EP” over “50” on one side, and “APO” on the other side. They are supplied as follows:
- Bottles of 30 (NDC 60505-2652-3)
- Bottles of 90 (NDC 60505-2652-9)
- Bottles of 1000 (NDC 60505-2652-8)
- Blisters of 100 (NDC 60505-2652-7)
Storage
- Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86° F).
- Dispense in a tight, light-resistant container.
Storage
There is limited information regarding Eplerenone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Eplerenone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eplerenone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients receiving eplerenone tablets should be informed:
- Not to use strong CYP3A4 inhibitors, such as ketoconazole, clarithromycin, nefazodone, ritonavir, and nelfinavir.
- Not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician.
- To call their physician if they experience dizziness, diarrhea, vomiting, rapid or irregular heartbeat, lower extremity edema, or difficulty breathing.
- That periodic monitoring of blood pressure and serum potassium is important.
Precautions with Alcohol
Alcohol-Eplerenone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Inspra®
Look-Alike Drug Names
- Inspra® — Spiriva®[6]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Pitt, Bertram; Remme, Willem; Zannad, Faiez; Neaton, James; Martinez, Felipe; Roniker, Barbara; Bittman, Richard; Hurley, Steve; Kleiman, Jay; Gatlin, Marjorie (2003). "Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction". New England Journal of Medicine. 348 (14): 1309–1321. doi:10.1056/NEJMoa030207. ISSN 0028-4793.
- ↑ Epstein, M. (2006). "Selective Aldosterone Blockade with Eplerenone Reduces Albuminuria in Patients with Type 2 Diabetes". Clinical Journal of the American Society of Nephrology. 1 (5): 940–951. doi:10.2215/CJN.00240106. ISSN 1555-9041.
- ↑ Weinberger, Myron H.; White, William B.; Ruilope, Luis-Miguel; MacDonald, Thomas M.; Davidson, Robert C.; Roniker, Barbara; Patrick, Jeffrey L.; Krause, Scott L. (2005). "Effects of eplerenone versus losartan in patients with low-renin hypertension". American Heart Journal. 150 (3): 426–433. doi:10.1016/j.ahj.2004.12.005. ISSN 0002-8703.
- ↑ Zannad, Faiez; McMurray, John J.V.; Krum, Henry; van Veldhuisen, Dirk J.; Swedberg, Karl; Shi, Harry; Vincent, John; Pocock, Stuart J.; Pitt, Bertram (2011). "Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms". New England Journal of Medicine. 364 (1): 11–21. doi:10.1056/NEJMoa1009492. ISSN 0028-4793.
- ↑ Swedberg, Karl; Zannad, Faiez; McMurray, John J.V.; Krum, Henry; van Veldhuisen, Dirk J.; Shi, Harry; Vincent, John; Pitt, Bertram (2012). "Eplerenone and Atrial Fibrillation in Mild Systolic Heart Failure". Journal of the American College of Cardiology. 59 (18): 1598–1603. doi:10.1016/j.jacc.2011.11.063. ISSN 0735-1097.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Eplerenone |Pill Name=Eplerenone018.jpg |Drug Name=Inspra 25 MG Oral Tablet |Pill Ingred=Eplerenone|+sep=; |Pill Imprint=Pfizer;NSR;25 |Pill Dosage=25 mg |Pill Color=Yellow|+sep=; |Pill Shape=Diamond |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=G.D. Searle LLC Division of Pfizer Inc |NDC=0025-1710
}}
{{#subobject:
|Page Name=Eplerenone |Pill Name=Eplerenone019.jpg |Drug Name=Eplerenone 25 MG Oral Tablet |Pill Ingred=Eplerenone|+sep=; |Pill Imprint=G;25mg |Pill Dosage=25 mg |Pill Color=Yellow|+sep=; |Pill Shape=Diamond |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Greenstone LLC |NDC=59762-1710
}}
{{#subobject:
|Page Name=Eplerenone |Pill Name=Eplerenone020.jpg |Drug Name=Inspra 50 MG Oral Tablet |Pill Ingred=Eplerenone|+sep=; |Pill Imprint=Pfizer;NSR;50 |Pill Dosage=50 mg |Pill Color=Yellow|+sep=; |Pill Shape=Diamond |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=G.D. Searle LLC Division of Pfizer Inc |NDC=0025-1720
}}
{{#subobject:
|Page Name=Eplerenone |Pill Name=Eplerenone021.jpg |Drug Name=Eplerenone 50 MG Oral Tablet |Pill Ingred=Eplerenone|+sep=; |Pill Imprint=G;50mg |Pill Dosage=50 mg |Pill Color=Yellow|+sep=; |Pill Shape=Diamond |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Greenstone LLC |NDC=59762-1720
}}
{{#subobject:
|Label Page=Eplerenone |Label Name=Eplerenone015.jpg
}}
{{#subobject:
|Label Page=Eplerenone |Label Name=Eplerenone016.jpg
}}
{{#subobject:
|Label Page=Eplerenone |Label Name=Eplerenone017.jpg
}}