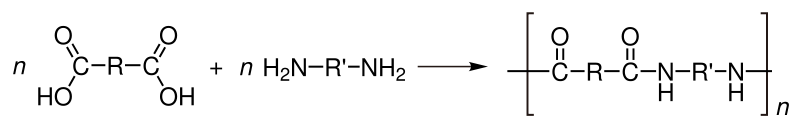Hydrolysis
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Hydrolysis is a chemical reaction or process in which a chemical compound is broken down by reaction with water.[1][2] It is the type of reaction that is used to break down certain polymers, especially those made by step-growth polymerization. Such polymer degradation is usually catalysed by either acid or alkali, attack often increasing with strength or pH.
Types
In organic chemistry, hydrolysis can be considered as the reverse or opposite of condensation, a reaction in which two molecular fragments are joined for each water molecule produced. As hydrolysis may be a reversible reaction, condensation and hydrolysis can take place at the same time, with the position of equilibrium determining the amount of each product.
In inorganic chemistry, the word is often applied to solutions of salts and the reactions by which they are converted to new ionic species or to precipitates (oxides, hydroxides, or salts). The addition of a molecule of water to a chemical compound, without forming any other products is usually known as hydration, rather than hydrolysis.
In biochemistry, hydrolysis is considered the reverse or opposite of dehydration synthesis. In hydrolysis, a water molecule (H2O), is added, whereas in dehydration synthesis, a molecule of water is removed.
In electrochemistry, hydrolysis can also refer to the electrolysis of water. In hydrolysis, a voltage is applied across an aqueous medium, which produces a current and breaks the water into its constituents, hydrogen and oxygen.
In polymer chemistry, hydrolysis of polymers can occur during high-temperature processing such as injection moulding leading to chain degradation and loss of product integrity. Polymers most at risk include PET, polycarbonate, nylon and other polymers made by step-growth polymerization. Such materials must be dried prior to moulding.
Hydrolysis of amide links
In other hydrolysis reactions, such as hydrolysis of an amide link into a carboxylic acid and an amine product or ammonia, only the carboxylic acid product has a hydroxyl group derived from the water. The amine product (or ammonia) gains the remaining hydrogen ion. A more specific case of the hydrolysis of an amide link is hydrolyzing the peptide links of amino acids.
Many polyamide polymers such as nylon 6,6 are attacked and hydrolysed by strong acids. Such attack leads to depolymerization and nylon products fail by fracturing when exposed to even small amounts of acid. The reaction is essentially the reverse of the synthesis from monomers:
Other polymers made by step-growth polymerization are susceptible to similar polymer degradation reactions. The problem is known as stress corrosion cracking.
Hydrolysis of metal salts
(As noted above, hydrolysis of metal salts is more commonly known as hydration.) Many metal ions are strong Lewis acids, and in water they may undergo hydrolysis to form basic salts. Such salts contain a hydroxyl group that is directly bound to the metal ion in place of a water ligand. The positive charge on metal ions creates an attraction to water, a Lewis base with a non-binding electron pair on the oxygen atom, and alters water's electron density. This in turn increases the polarity of the O-H bond, which now acts as a proton donor under Brønsted-Lowry acid-base theory to release the hydrogen as a H+ ion, increasing the acidity of the solution. For example, aluminium chloride undergoes extensive hydrolysis in water such that the solution becomes very acidic.
This implies that hydrogen chloride is lost in the evaporation of AlCl3 solutions and the residue is a basic salt (in this case an an oxychloride) in place of AlCl3. Such behaviour is also seen with other metal chlorides such as ZnCl2, SnCl2, FeCl3 and lanthanide halides such as DyCl3. With some compounds such as TiCl4, the hydrolysis may go to completion and form the pure hydroxide or oxide, in this case TiO2.
Hydrolysis of cellulose (Cellulolysis)
Cellulolytic is relating to or causing the hydrolysis of cellulose (i.e. cellulolytic bacteria, fungi or enzymes).
The hydrolysis into glucose (i.e. of cellulose or starch) is called saccharification.
Irreversibility of hydrolysis under physiological conditions
Under physiological conditions (i.e. in dilute aqueous solution), a hydrolytic cleavage reaction, where the concentration of a metabolic precursor is low (on the order of 10-3 to 10-6 molar), is essentially thermodynamically irreversible. To give an example:
- A + H2O → X + Y
- <math>K_d = \frac{\left[X\right] \left[Y\right]} {\left[H_2O\right] \left[A\right]}</math>
Assuming that x is the final concentration of products, and that C is the initial concentration of A, and W = [H2O] = 55.5 molar, then x can be calculated with the equation:
- <math>\frac{x \times x}{W\left(C - x\right)} = K_d</math>
let Kd×W = k:
then <math> x = \frac {-k + \sqrt {k^2 + 4kC} } {2}. </math>
For a value of C = 0.001 molar, and k = 1 molar, x/C > 0.999. Less than 0.1% of the original reactant would be present once the reaction is complete.
This theme of physiological irreversibility of hydrolysis is used consistently in metabolic pathways, since many biological processes are driven by the cleavage of anhydrous pyrophosphate bonds.
See also
References
- ↑ Compendium of Chemical Terminology, hydrolysis, accessed 2007-01-23.
- ↑ Compendium of Chemical Terminology, solvolysis, accessed 2007-01-23.
External links
| Wikimedia Commons has media related to Collagen. |
bs:Hidroliza
bg:Хидролиза
ca:Hidròlisi
cs:Hydrolýza
da:Hydrolyse
de:Hydrolyse
eo:Hidrolizo
ko:가수분해
it:Idrolisi
he:הידרוליזה
lv:Hidrolīze
lt:Hidrolizė
mk:Хидролиза
nl:Hydrolyse
no:Hydrolyse
sq:Hidrolizë
simple:Hydrolysis
sk:Hydrolýza
sr:Хидролиза
sh:Hidroliza
su:Hidrolisis
fi:Hydrolyysi
sv:Hydrolys
uk:Гідроліз