Human ATP synthase
Editor In-Chief: Henry A. Hoff
Overview
A typical adult human utilizes ~50 kg of ATP per day under normal activity levels, requiring roughly a 1000-fold turnover of the 50 g of ATP/ADP present in the body.
- ADP + Pi <=> ATP + H2O : Enzyme ATPase/ATP synthase.
ATPase
ATPases are a class of enzymes that catalyze the decomposition of adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and a free phosphate ion. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Proton-translocating ATPases have fundamental roles in energy conservation, secondary active transport, acidification of intracellular organelles, and cellular pH homeostasis.
There are four classes of ATPases: E, F, P, and V. The F and V classes contain rotary motors.
E-ATPases are cell-surface enzymes that hydrolyse a range of NTPs, including extracellular ATP.
- Tentative, Mg2+ transporting: ATP3
F-ATPase (F1FO-ATPase) can use energy released by ATP hydrolysis to pump protons against their thermodynamic gradient.
- H+ transporting, mitochondrial: ATP5A1, ATP5B, ATP5C1, ATP5C2, ATP5D, ATP5E, ATP5F1, ATP5G1, ATP5G2, ATP5G3, ATP5H, ATP5I, ATP5J, ATP5J2, ATP5L, ATP5L2, ATP5O, ATP5S
P-ATPases are found in the plasma membranes and organelles, and function to transport a variety of different ions across membranes.
- Amphipaths, such as phosphatidylserine, transporting, Class I, type 8: ATP8A1, ATP8B1, ATP8B2, ATP8B3, ATP8B4
- Cation transporting, phosphatidylserine and phosphatidylethanolamine transporting, Class V, type 10: ATP10A, ATP10B, ATP10D
- H+/K+ transporting, nongastric: ATP12A
V-ATPases (V1VO-ATPases) are primarily found in vacuoles, catalysing ATP hydrolysis to transport solutes and lower pH in organelles. The vacuolar (V-type) ATPases have a transmembrane proton-conducting sector and an extramembrane catalytic sector.
- H+ transporting, lysosomal: ATP6AP1, ATP6AP2, ATP6V1A, ATP6V1B1, ATP6V1B2, ATP6V1C1, ATP6V1C2, ATP6V1D, ATP6V1E1, ATP6V1E2, ATP6V1F, ATP6V1G1, ATP6V1G2, ATP6V1G3, ATP6V1H, ATP6V0A1, ATP6V0A2, ATP6V0A4, ATP6V0B, ATP6V0C, ATP6V0D1, ATP6V0D2, ATP6V0E
ATP synthase
An ATP synthase (EC 3.6.3.14) is a general term for an enzyme that can synthesize adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and inorganic phosphate by using some form of energy.
There are four classes of ATP synthase: E, F, P, and V. Each is as described above under ATPase, but operating in reverse.
E-ATP synthase
F-ATP synthase
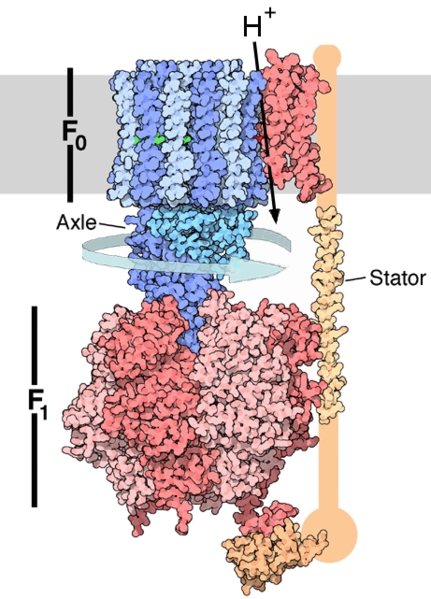
The great majority of ATP is produced by F-ATP synthases (F1FO-ATP synthase) in mitochondrial membranes. F-ATP synthase is an anabolic enzyme that harnesses the energy of a transmembrane electrochemical gradient of protons across the inner membrane generated by oxidative phosphorylation as an energy source for adding an inorganic phosphate group to ADP to form ATP.

This enzyme works when a proton moves down the concentration gradient, giving the enzyme a spinning motion. The rotor rotates in the membrane plane.[1] This unique spinning motion bonds ADP and Pi together to create ATP.
This ATP synthase is composed of two linked multi-subunit complexes: the soluble catalytic core, F1, and the membrane-spanning component, FO, comprising the proton channel. The catalytic portion of mitochondrial ATP synthase consists of 5 different subunits (alpha, beta, gamma, delta, and epsilon) assembled with a stoichiometry of 3 alpha, 3 beta, and a single representative each of the other 3. The general proton channel consists of three main subunits (a, b, c) plus d, e, f, g, h. The ring of rotating proteins consists of 10, 11 or 14 subunit c proteins.
The human proton channel seems to have nine subunits (a, b, c, d, e, f, g, F6 and 8).[2][3][4]
The mammalian form of this enzyme has been extensively studied using beef heart and rat liver as a source. It is a complex of 16 different subunits with F1: alpha, beta, gamma, delta, epsilon and FO: a, b, c, d, e, f, g, A6L, O subunit, and coupling factor 6 (F6). Associated in the complex under some conditions is an intrinsic inhibitor protein IF1. Human F1FO ATP synthase has been characterized from human heart. The enzyme has the same subunit composition as that from bovine heart.[5] The associated inhibitor protein IF1 is the product of gene EIF1AX GeneID: 1964[6].
Human F complex:
Catalytic core (F1 - Fraction 1)
alpha subunit: ATP5A1 GeneID: 498[7] ATPAF2 GeneID: 91467[8].
beta subunit: ATP5B GeneID: 506[9] ATPAF1 GeneID: 64756[10] ATP5BL1 GeneID: 507[11] ATP5BL2 GeneID: 508[12] C16orf7 GeneID: 9605[13]. ATP synthesis coupled proton transport.
gamma subunit: ATP5C1 GeneID: 509[14].
delta subunit: The delta complex comprises the peripheral stator that is bound to F0 subunit a, and to the F1 hexamer (3 alpha, 3 beta).[15] ATP5D GeneID: 513[16].
epsilon subunit: ATP5E GeneID: 514[17].
Human proton channel (membrane-spanning component, FO)
subunit a: Subunit 6 ATP6 GeneID: 4508[18] is the homolog of subunit a.[19] The delta complex interacts with subunit a. Subunit a forms the complex ab2 with the subunit b dimer. Together with F1 components alpha(3), beta(3), and delta form the stator of ATP synthase.
subunit b: This subunit peripherally connects by multiple contacts with alpha, beta and gamma subunits, and with subunit a. ATP5F1 GeneID: 515[20] LOC100128516 GeneID: 100128516[21]. According to automated computational analysis this gene may be transcribed to a miscRNA involved herein.
subunit c: Together with the gamma and epsilon subunits, subunit c forms the centrally located rotor element[22] which has to be counteracted by a peripheral stator element. ATP5G1 GeneID: 516[23] ATP5G2 GeneID: 517[24] ATP5G3 GeneID: 518[25] LOC728205 GeneID: 728205[26]. Computer modeling of this gene suggests that it may be transcribed as a miscRNA herein involved. LOC100130492 GeneID: 100130492[27]. Computer modeling of this gene suggests that it may be transcribed as a miscRNA herein involved.
subunit d: ATP5H GeneID: 10476[28].
subunit e: ATP5I (ATP5K) GeneID: 521[29].
subunit f: ATP5J2 GeneID: 9551[30] LOC645225 GeneID: 645225[31].
subunit g: ATP5L GeneID: 10632[32].
subunit A6L: Fungal ATP synthase FO subunit 8 (A6L) is an EC:3.6.3.14 and may be related to human ATP synthase protein 8 (subunit 8).[33] Subunit 8 is ATP8 GeneID: 4509[34]. Subunit 8 possesses a single transmembrane domain which extends across the inner membrane of intact mitochondria. As subunit d is a likely component of the stator stalk and there are cross-links between subunits 8 and d, subunit 8 is a part of the stator stalk.[35][36]
Connector (links catalytic core with proton channel):
subunit O (OSCP - the oligomycin sensitivity conferral protein): ATP5O GeneID: 539[37]. ATP5O may be part of the connector linking F1 and FO components and may be involved in transmission of conformational changes or proton conductance.[38].
subunit F6: ATP5J GeneID: 522[39].
subunit s: ATP5S GeneID: 27109[40]. But others have not found this subunit s (Factor B) to be present in FO.[41] ATP5SL GeneID: 55101[42].
References
- ↑ Kaim G and Dimroth P (1998). "Voltage-generated torque drives the motor of the ATP synthase". EMBO J. 17 (20): 5887–5896. doi:10.1093/emboj/17.20.5887. Text "pmid: 9774333 " ignored (help); Unknown parameter
|month=ignored (help) - ↑ "ATP5F1 ATP synthase, H+ transporting, mitochondrial FO complex, subunit B1".
- ↑ "ATP5G1 ATP synthase, H+ transporting, mitochondrial FO complex, subunit C1 (subunit 9)".
- ↑ "ATP5H ATP synthase, H+ transporting, mitochondrial FO complex, subunit d".
- ↑ Aggeler R, Coons J, Taylor SW, Ghosh SS, Garcia JJ, Capaldi RA, Marusich MF (2002). "A functionally active human F1F0 ATPase can be purified by immunocapture from heart tissue and fibroblast cell lines. Subunit structure and activity studies". J Biol Chem. 277 (37): 33906–33912. doi:10.1074/jbc.M204538200. Text "pmid: 12110673 " ignored (help); Unknown parameter
|month=ignored (help) - ↑ "EIF1AX eukaryotic translation initiation factor 1A, X-linked".
- ↑ "Entrez Gene: ATP5A1 ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit 1, cardiac muscle".
- ↑ "Entrez Gene: ATPAF2 ATP synthase mitochondrial F1 complex assembly factor 2".
- ↑ "Entrez Gene: ATP5B ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide".
- ↑ "Entrez Gene: ATPAF1 ATP synthase mitochondrial F1 complex assembly factor 1".
- ↑ "Entrez Gene: ATP5BL1 ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide-like 1".
- ↑ "Entrez Gene: ATP5BL2 ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide-like 2".
- ↑ "Entrez Gene: C16orf7 chromosome 16 open reading frame 7".
- ↑ "Entrez Gene: ATP5C1 ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1".
- ↑ Krebstakies T, Zimmermann B, Gräber P, Altendorf K, Börsch M, Greie JC (2005). "Both rotor and stator subunits are necessary for efficient binding of F1 to F0 in functionally assembled Escherichia coli ATP Synthase". J Biol Chem. 280 (39): 33338–45. doi:10.1074/jbc.M506251200. PMID 16085645. Unknown parameter
|month=ignored (help) - ↑ "Entrez Gene: ATP5D ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit".
- ↑ "Entrez Gene: ATP5E ATP synthase, H+ transporting, mitochondrial F1 complex, epsilon subunit".
- ↑ "Entrez Gene: ATP6 ATP synthase FO subunit 6".
- ↑ Stephens AN, Khan MA, Roucou X, Nagley P, Devenish RJ (2003). "The molecular neighborhood of subunit 8 of yeast mitochondrial F1FO-ATP synthase probed by cysteine scanning mutagenesis and chemical modification". J Biol Chem. 278 (20): 17867–75. Text "pmid: 12626501 " ignored (help); Text "doi:10.1074/jbc.M300967200" ignored (help); Unknown parameter
|month=ignored (help) - ↑ "Entrez Gene: ATP5F1 ATP synthase, H+ transporting, mitochondrial F0 complex, subunit B1".
- ↑ "Entrez Gene: LOC100128516 similar to ATP synthase, H+ transporting, mitochondrial FO complex, subunit B1".
- ↑ Amzel LM, Bianchet MA, Leyva JA (2003). "Understanding ATP synthesis: structure and mechanism of the F1-ATPase (Review)". Mol. Membr. Biol. 20 (1): 27–33. doi:10.1080/0968768031000066532. PMID 12745923.
- ↑ "Entrez Gene: ATP5G1 ATP synthase, H+ transporting, mitochondrial FO complex, subunit C1 (subunit 9)".
- ↑ "Entrez Gene: ATP5G2 ATP synthase, H+ transporting, mitochondrial FO complex, subunit C2 (subunit 9)".
- ↑ "Entrez Gene: ATP5G3 ATP synthase, H+ transporting, mitochondrial F0 complex, subunit C3 (subunit 9)".
- ↑ "Entrez Gene: LOC728205 similar to P1 gene for c subunit of human mitochondrial ATP synthase".
- ↑ "Entrez Gene: LOC100130492 similar to P1 gene for c subunit of human mitochondrial ATP synthase".
- ↑ "Entrez Gene: ATP5H ATP synthase, H+ transporting, mitochondrial FO complex, subunit d".
- ↑ "Entrez Gene: ATP5I ATP synthase, H+ transporting, mitochondrial FO complex, subunit E".
- ↑ "Entrez Gene: ATP5J2 ATP synthase, H+ transporting, mitochondrial FO complex, subunit F2".
- ↑ "Entrez Gene: LOC645225 similar to ATP synthase, H+ transporting, mitochondrial FO complex, subunit F2".
- ↑ "Entrez Gene: ATP5L ATP synthase, H+ transporting, mitochondrial FO complex, subunit G".
- ↑ "Entrez Gene: ATP5O ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit".
- ↑ "Entrez Gene: ATP8 ATP synthase FO subunit 8".
- ↑ Stephens AN, Khan MA, Roucou X, Nagley P, Devenish RJ (2003). "The molecular neighborhood of subunit 8 of yeast mitochondrial F1FO-ATP synthase probed by cysteine scanning mutagenesis and chemical modification". J Biol Chem. 278 (20): 17867–75. Text "pmid: 12626501 " ignored (help); Text "doi:10.1074/jbc.M300967200" ignored (help); Unknown parameter
|month=ignored (help) - ↑ Stephens AN, Roucou X, Artika IM, Devenish RJ, Nagley P (2000). "Topology and proximity relationships of yeast mitochondrial ATP synthase subunit 8 determined by unique introduced cysteine residues". Eur J Biochem. 267 (21): 6443–51. Text "pmid: 11029588 " ignored (help); Unknown parameter
|month=ignored (help) - ↑ "Entrez Gene: ATP5O ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit".
- ↑ "Entrez Gene: ATP5O ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit".
- ↑ "Entrez Gene: ATP5J ATP synthase, H+ transporting, mitochondrial F0 complex, subunit F6".
- ↑ "Entrez Gene: ATP5S ATP synthase, H+ transporting, mitochondrial FO complex, subunit s (factor B)".
- ↑ Aggeler R, Coons J, Taylor SW, Ghosh SS, Garcia JJ, Capaldi RA, Marusich MF (2002). "A functionally active human F1FO ATPase can be purified by immunocapture from heart tissue and fibroblast cell lines. Subunit structure and activity studies". J Biol Chem. 277 (37): 33906–33912. doi:10.1074/jbc.M204538200. Text "pmid: 12110673 " ignored (help); Unknown parameter
|month=ignored (help) - ↑ "Entrez Gene: ATP5SL ATP5S-like".