Heteropoly acid
A heteropoly acid is a class of acid made up of a particular combination of hydrogen and oxygen with certain metals and non-metals. This type of acid is frequently used as a re-usable acid catalyst in chemical reactions.
To qualify as a heteropoly acid, the compound must contain:
- a metal such as tungsten, molybdenum or vanadium, termed the addenda atom;
- oxygen;
- an element generally from the p-block of the periodic table, such as silicon, phosphorus or arsenic termed the hetero atom;
- acidic hydrogen atoms.
The metal addenda atoms linked by oxygen atoms form a cluster with the hetero-atom inside bonded via oxygen atoms. Examples with more than one type of metal addenda atom in the cluster are well known. The conjugate anion of a heteropoly acid is known as a polyoxometalate.
Due to the possibilities of there being different combinations of addenda atoms and different types of hetero atoms there are a lot of heteropolyacids. Two of the better known groups of these are based on the Keggin, HnXM12O40, and Dawson, HnX2M18O62, structures.
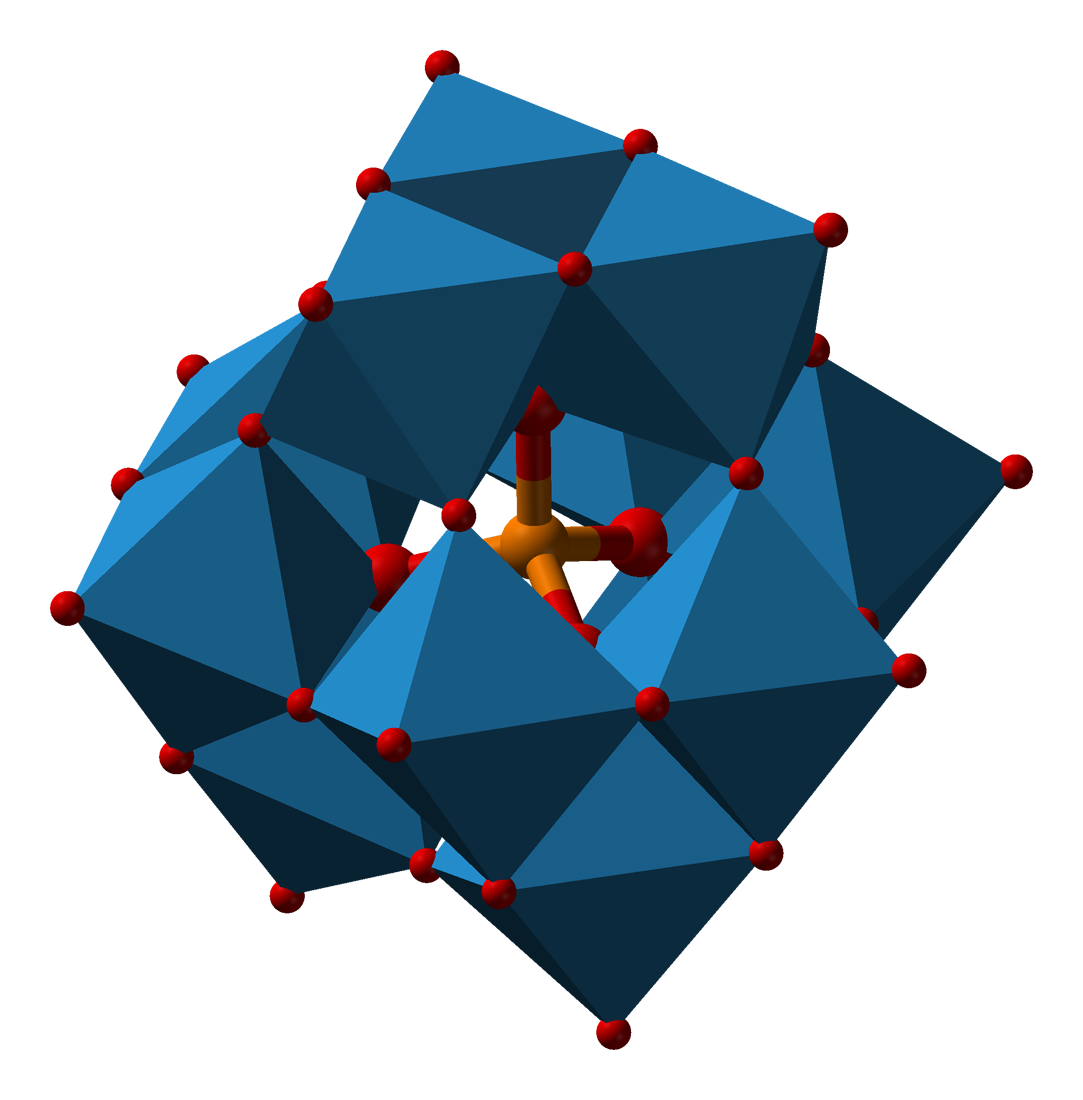
|
Dawson ion |
| Keggin structure, XM12O40n− | Dawson structure, X2M18O62n− |
Some examples are:
- H4Xn+M12O40, X = Si, Ge; M = Mo, W
- H3Xn+M12O40, X = P, As; M = Mo, W
- H6X2M18O62, X=P, As;M = Mo, W
The heteropolyacids are widely used as homogeneous and heterogeneous catalysts, particularly those based on the Keggin structure as they can possess qualities such as good thermal stability, high acidity and high oxidising ability. Some examples of catalysis are[1]:
- Homogeneous acid catalysis
- hydrolysis of propene to give propan-2-ol by H3PMo12O40 and H3PW12O40
- Prins reaction by H3PW12O40
- polymerisation of THF by H3PW12O40
- Heterogeneous acid catalysis
- dehydration of propan-2-ol to propene and methanol to hydrocarbons by H3PW12O40
- reformation of hexane to 2-methylpentane (isohexane) by H3PW12O40 on SiO2
- Homogeneous oxidation
- cyclohexene + H2O2 to adipic acid by the mixed addenda H3PMo6V6O40
- ketone by O2 to acid and aldehyde by mixed addenda H5PMo10V2O40
Heteropolyacids have long been used in analysis and histology and are a component of many reagents e.g. the Folin-Ciocalteu reagent, folins phenol reagent used in the Lowry protein assay and EPTA, ethanolic phosphotungstic acid.
See also
References
- ↑ Oxide catalysts in solid state chemistry T Okuhara, M Misono Encyclopedia of Inorganic chemistry Editor R Bruce King (1994) John Wiley and Sons ISBN 0471 93620 0