Disulfide bond
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
In chemistry, a disulfide bond is a single covalent bond derived from the coupling of thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore C-S-S-C. The terminology is almost exclusively used in biochemistry, bioinorganic and bioorganic chemistry. Formally the connection is called a persulfide, in analogy to its congener, a peroxide (R-O-O-R), but this terminology is rare.
Three sulfur atoms singly bonded in a sequence are sometimes called a trisulfide bond, although there are in fact two S-S bonds. Disulfide bonds are usually formed from the oxidation of sulfhydryl (-SH) groups, as depicted formally in Figure 1.

Disulfide bonds in proteins
Disulfide bonds play an important role in the folding and stability of some proteins, usually proteins secreted to the extracellular medium. Since most cellular compartments are a reducing environment, disulfide bonds are generally unstable in the cytosol (with some exceptions noted below).
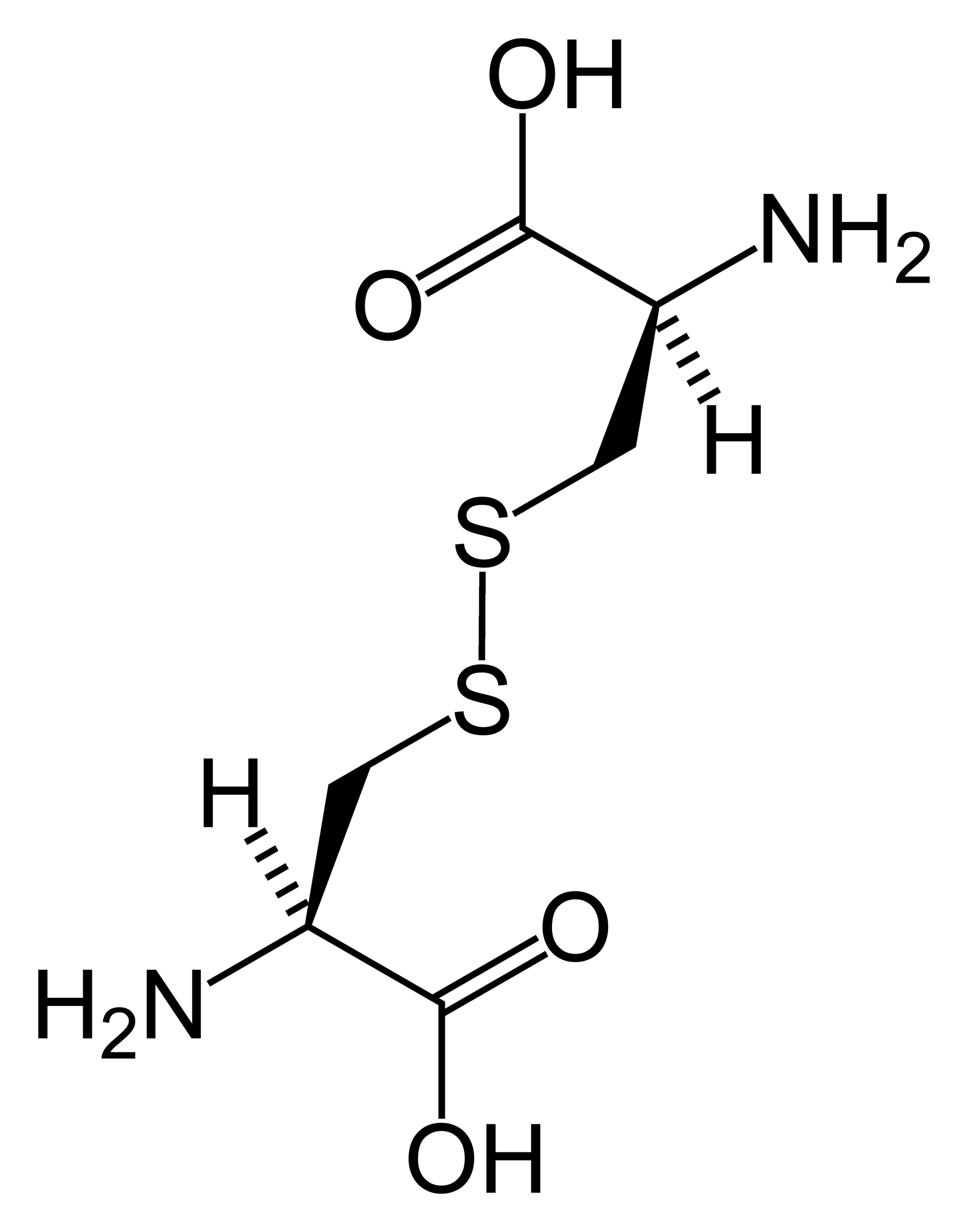
Disulfide bonds in proteins are formed between the thiol groups of cysteine residues. The other sulfur-containing amino acid, methionine, cannot form disulfide bonds. A disulfide bond is typically denoted by hyphenating the abbreviations for cysteine, e.g., the "Cys26-Cys84 disulfide bond", or the "26-84 disulfide bond", or most simply as "C26-C84" where the disulfide bond is understood and does not need to be mentioned. The prototype of a protein disulfide bond is the two-amino-acid peptide, cystine, which is composed of two cysteine amino acids joined by a disulfide bond (shown in Figure 2 in its unionized form). The structure of a disulfide bond can be described by its <math>\chi_{ss}</math> dihedral angle between the <math>C^{\beta}-S^{\gamma}-S^{\gamma}-C^{\beta}</math> atoms, which is usually close to ±90°.
The disulfide bond stabilizes the folded form of a protein in several ways: 1) It holds two portions of the protein together, biasing the protein towards the folded topology. Expressed differently, the disulfide bond destabilizes the unfolded form of the protein by lowering its entropy. 2) The disulfide bond may form the nucleus of a hydrophobic core of the folded protein, i.e., local hydrophobic residues may condense around the disulfide bond and onto each other through hydrophobic interactions. 3) Related to #1 and #2, the disulfide bond link two segments of the protein chain, the disulfide bond increases the effective local concentration of protein residues and lowers the effective local concentration of water molecules. Since water molecules attack amide-amide hydrogen bonds and break up secondary structure, a disulfide bond stabilizes secondary structure in its vicinity. For example, researchers have identified several pairs of peptides that are unstructured in isolation, but adopt stable secondary and tertiary structure upon forming a disulfide bond between them.
Disulfide bonds in proteins are formed by thiol-disulfide exchange reactions. A disulfide species is a particular pairing of cysteines in a disulfide-bonded protein and is usually depicted by listing the disulfide bonds in parentheses, e.g., the "(26-84, 58-110) disulfide species". A disulfide ensemble is a grouping of all disulfide species with the same number of disulfide bonds, and is usually denoted as the 1S ensemble, the 2S ensemble, etc. for disulfide species having one, two, etc. disulfide bonds. Thus, the (26-84) disulfide species belongs to the 1S ensemble, whereas the (26-84, 58-110) species belongs to the 2S ensemble. The single species with no disulfide bonds is usually denoted as R for "fully reduced". Under typical conditions, disulfide reshuffling is much faster than the formation of new disulfide bonds or their reduction; hence, the disulfide species within an ensemble equilibrate more quickly than between ensembles.
The native form of a protein is usually a single disulfide species, although some proteins may cycle between a few disulfide states as part of their function, e.g., thioredoxin. In proteins with more than two cysteines, non-native disulfide species may be formed, which are almost always unfolded. As the number of cysteines increases, the number of nonnative species increases factorially. The number of ways of forming p disulfide bonds from n cysteine residues is given by the formula
- <math>
\frac{n!}{p! \ (n - 2p)! \ 2^{p}} </math>
For example, an eight-cysteine protein such as ribonuclease A has 105 different four-disulfide species, only one of which is the native disulfide species. Isomerases have been identified that catalyze the interconversion of disulfide species, accelerating the formation of the native disulfide species.
Disulfide species that have only native disulfide bonds (but not all of them) are denoted by des followed by the lacking native disulfide bond(s) in square brackets. For example, the des[40-95] disulfide species has all the native disulfide bonds except that between cysteines 40 and 95. Disulfide species that lack one native disulfide bond are frequently folded, particularly if the missing disulfide bond is exposed to solvent in the folded, native protein.
In order to analyze the structure of proteins, it is often necessary to break disulfide bonds. This reduction of disulfide bonds can be accomplished by treatment with 2-Mercaptoethanol.
In prokaryotes
Disulfide bonds play an important protective role for bacteria as a reversible switch that turns a protein on or off when bacterial cells are exposed to oxidation reactions. Hydrogen peroxide (H2O2) in particular could severely damage DNA and kill the bacterium at low concentrations if not for the protective action of the SS-bond.
In rubber
Disulfide bonds also play a significant role in the vulcanization of rubber.
In eukaryotes
In eukaryotic cells, disulfide bonds are generally formed in the lumen of the RER (rough endoplasmic reticulum) but not in the cytosol. This is due to the oxidative environment of the ER and the reducing environment of the cytosol (see glutathione). Thus disulfide bonds are mostly found in secretory proteins, lysosomal proteins, and the exoplasmic domains of membrane proteins.
There are notable exceptions to this rule. A number of cytosolic proteins have cysteine residues in proximity to each other that function as oxidation sensors; when the reductive potential of the cell fails, they oxidize and trigger cellular response mechanisms. Vaccinia virus also produces cytosolic proteins and peptides that have many disulfide bonds; although the reason for this is unknown presumably they have protective effects against intracellular proteolysis machinery.
Disulfide bonds are also formed within and between protamines in the sperm chromatin of many mammalian species.
In hair and feathers
Hair is a biological polymer, with over 90% of its dry weight made of proteins called keratins. Under normal conditions, human hair contains around 10% water, which modifies its mechanical properties considerably. Hair proteins are held together by disulfide bonds, from the amino acid cysteine. These links are very robust: for example, virtually intact hair has been recovered from ancient Egyptian tombs, and the disulfide links also cause hair (and feathers which have similar keratins) to be extremely resistant to protein digestive enzymes. Different parts of the hair and feather have different cysteine levels, leading to harder or softer material.
Breaking and making disulfide bonds governs the phenomenon of wavy or frizzy hair. It is breaking and remaking of the disulfide bonds which is the basis for the permanent wave in hairstyling.
In feathers, the high disulfide content dictates the high sulfur content of bird eggs, which need to contain enough sulfur to feather the chick.
In both hair and feathers, the high sulfur content due to the high number of disulfides causes the disagreeable smell of the material when it is burned.
In organic chemistry
The Zincke disulfide cleavage is a classic organic reaction in which a disulfide is converted to a sulfur halide R-S-X with X = Br, Cl by reaction with bromine or chlorine.[1][2][3][4][5] It was named after Theodor Zincke.
General references
- Sela M and Lifson S. (1959) "On the Reformation of Disulfide Bridges in Proteins", Biochimica et Biophysica Acta, 36, 471-478.
- Stark GR. (1977) "Cleavage at cysteine after cyanylation", Methods in Enzymology, 11, 238-255.
- Thornton JM. (1981) "Disulphide Bridges in Globular Proteins", Journal of Molecular Biology, 151, 261-287.
- Thannhauser TW, Konishi Y and Scheraga HA. (1984) "Sensitive Quantitative Analysis of Disulfide Bonds in Polypeptides and Proteins", Analytical Biochemistry, 138, 181-188.
- Wu J and Watson JT. (1998) "Optimization of the Cleavage Reaction for Cyanylated Cysteinyl Proteins for Eficient and Simplified Mas Mapping", Analytical Biochemistry, 258, 268-276.
- Futami J, Tada H, Seno M, Ishikami S and Yamada H. (2000) "Stabilization of Human RNase 1 by Introduction of a Disulfide Bond between Residues 4 and 118", J. Biochem., 128, 245-250.
References
- ↑ Zincke, Th. (1911). "Über eine neue Reihe aromatischer Schwefelverbindungen". Chemische Berichte. 44 (1): 769–771. doi:10.1002/cber.191104401109.
- ↑ Th. Zincke, Fr. Farr (1912). "Über o-Nitrophenylschwefelchlorid und Umwandlungsprodukte". Justus Liebig's Annalen der Chemie. 391 (1): 57–88. doi:10.1002/jlac.19123910106.
- ↑ for example the conversion of di-o-nitrophenyl disulfide to o-nitrophenylsulfur chloride Organic Syntheses, Coll. Vol. 2, p.455 (1943); Vol. 15, p.45 (1935) Link
- ↑ Related reactions : Organic Syntheses, Coll. Vol. 9, p.662 (1998); Vol. 74, p.124 (1997) Link
- ↑ Organic Syntheses, Coll. Vol. 5, p.709 (1973); Vol. 40, p.62 (1960) Link
External links
- Synthesis of Disulfides
- Disulfide bonds and hair
- Protein disulfide bond formation in prokaryotes
- Oxidative protein folding in eukaryotes : mechanisms and consequences
- The human protein disulfide isomerase family: substrate interactions and functional properties
cy:Bond deusylffid da:Disulfidbinding de:Disulfidbrücke ko:디설피드 결합 he:קשר דיסולפידי nl:Zwavelbrug fi:Disulfidisidos sv:Disulfidbindning