Amphetamine
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | α-methylphenethylamine |
| AHFS/Drugs.com | amphetamine |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Dependence liability | Moderate |
| Routes of administration | Medical: oral, nasal inhalation Recreational: oral, nasal inhalation, insufflation, rectal, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Rectal 95–100%; Oral 75–100%[9] |
| Protein binding | 15–40%[10] |
| Metabolism | CYP2D6,[1] DBH,[2][3][4] FMO3,[5][6] XM-ligase,[7] and ACGNAT[8] |
| Onset of action | Immediate |
| Elimination half-life | D-amph:9–11h;[1][11] L-amph:11–14h[1][11] |
| Excretion | Renal; pH-dependent range: 1–75%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C9H13N |
| Molar mass | 135.2084 g/mol |
| 3D model (JSmol) | |
| Density | 0.9±0.1 g/cm3 |
| Melting point | 11.3 °C (52.34 °F) [12] |
| Boiling point | 203 °C (397.4 °F) [13] |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Amphetamine is a potent central nervous system stimulant used in the treatment of attention deficit hyperactivity disorder and narcolepsy.
Amphetamine
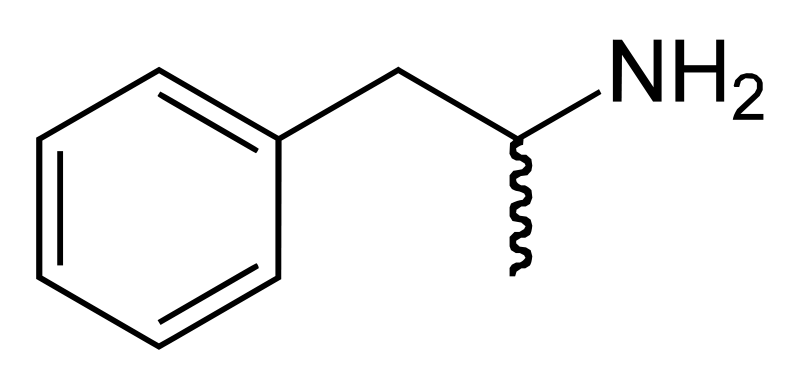
Amphetamine[note 1] (Lua error: expandTemplate: template "Template:IPA audio link" does not exist.; contracted from alpha‑methylphenethylamine) is a potent central nervous system (CNS) stimulant of the phenethylamine class that is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. Amphetamine was discovered in 1887 and exists as two enantiomers: levoamphetamine and dextroamphetamine.[note 2] Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers, levoamphetamine and dextroamphetamine, in their pure amine forms. However, the term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion, depression, and obesity. Amphetamine is also used as a performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription medication in many countries, and unauthorized possession and distribution of amphetamine is often tightly controlled due to the significant health risks associated with uncontrolled or heavy use.[sources 1]
The first pharmaceutical amphetamine was Benzedrine, a brand of inhalers used to treat a variety of conditions. Currently, pharmaceutical amphetamine is typically prescribed as Adderall,[note 3] dextroamphetamine, or the inactive prodrug lisdexamfetamine. Amphetamine, through activation of a trace amine receptor, increases biogenic amine and excitatory neurotransmitter activity in the brain, with its most pronounced effects targeting the catecholamine neurotransmitters norepinephrine and dopamine. At therapeutic doses, this causes emotional and cognitive effects such as euphoria, change in libido, increased wakefulness, and improved cognitive control. It induces physical effects such as decreased reaction time, fatigue resistance, and increased muscle strength.[sources 2]
Much larger doses of amphetamine are likely to impair cognitive function and induce rapid muscle breakdown. Drug addiction is a serious risk of amphetamine abuse, but only rarely arises from medical use. Very high doses can result in psychosis (e.g., delusions and paranoia) which rarely occurs at therapeutic doses even during long-term use. Recreational doses are generally much larger than prescribed therapeutic doses, and carry a far greater risk of serious side effects.[sources 3]
Amphetamine is also the parent compound of its own structural class, the substituted amphetamines,[note 4] which includes prominent substances such as bupropion, cathinone, MDMA (ecstasy), and methamphetamine. Unlike methamphetamine, amphetamine's salts lack sufficient volatility to be smoked. As a member of the phenethylamine class, amphetamine is also chemically related to the naturally occurring trace amine neuromodulators, specifically phenethylamine[note 5] and N-methylphenethylamine, both of which are produced within the human body.[sources 4]
Uses
Medical
Amphetamine is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy, and is sometimes prescribed off-label for its past medical indications, such as depression, obesity, and nasal congestion.[11][27] Long-term amphetamine exposure in some animal species is known to produce abnormal dopamine system development or nerve damage,[38][39] but, in humans with ADHD, amphetamines appear to improve brain development and nerve growth.[40][41][42] Magnetic resonance imaging studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus.[40][41][42]
Reviews of clinical stimulant research have established the safety and effectiveness of long-term amphetamine use for ADHD.[43][44] Controlled trials spanning two years have demonstrated treatment effectiveness and safety.[44][45] One review highlighted a nine-month randomized controlled trial in children with ADHD that found an average increase of 4.5 IQ points and continued improvements in attention, disruptive behaviors, and hyperactivity.[45]
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems;[46] these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the locus coeruleus and prefrontal cortex.[46] Psychostimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems.[24][46][47] Approximately 70% of those who use these stimulants see improvements in ADHD symptoms.[48][49] Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans.[43][48] The Cochrane Collaboration's review[note 6] on the treatment of adult ADHD with amphetamines stated that while amphetamines improve short-term symptoms, they have higher discontinuation rates than non-stimulant medications due to their adverse side effects.[51]
A Cochrane Collaboration review on the treatment of ADHD in children with tic disorders indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.[52] Other Cochrane reviews on the use of amphetamine following stroke or acute brain injury indicated that it may improve recovery, but further research is needed to confirm this.[53][54][55]
Enhancing performance
Therapeutic doses of amphetamine improve cortical network efficiency, resulting in higher performance on working memory tests in all individuals.[24][56] Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior.[24][57][58] Stimulants such as amphetamine can improve performance on difficult and boring tasks,[24][57] and are used by some students as a study and test-taking aid.[59] Based upon studies of self-reported illicit stimulant use, performance-enhancing use, rather than abuse as a recreational drug, is the primary reason that students use stimulants.[60] However, high amphetamine doses that are above the therapeutic range can interfere with working memory and cognitive control.[24][57]
Amphetamine is used by some athletes for its psychological and performance-enhancing effects, such as increased stamina and alertness;[23][35] however, its use is prohibited at sporting events regulated by collegiate, national, and international anti-doping agencies.[61][62] In healthy people at oral therapeutic doses, amphetamine has been shown to increase physical strength, acceleration, stamina, and endurance, while reducing reaction time.[23][63][64] Amphetamine improves stamina, endurance, and reaction time primarily through reuptake inhibition and effluxion of dopamine in the central nervous system.[63][64][65] At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance;[23][63][64] however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.[22][31][63]
Contraindications
According to the International Programme on Chemical Safety (IPCS) and United States Food and Drug Administration (USFDA),[note 7] amphetamine is contraindicated in people with a history of drug abuse, heart disease, severe agitation, or severe anxiety.[66][67] It is also contraindicated in people currently experiencing arteriosclerosis (hardening of the arteries), glaucoma (an eye condition), hyperthyroidism (excessive production of thyroid hormone), or hypertension (elevated blood pressure).[66][67] People who have experienced allergic reactions to other stimulants in the past or are taking monoamine oxidase inhibitors (MAOIs) are advised not to take amphetamine.[66][67] These agencies also state that anyone with anorexia nervosa, bipolar disorder, depression, elevated blood pressure, liver or kidney problems, mania, psychosis, Raynaud's phenomenon, seizures, thyroid problems, tics, or Tourette syndrome should monitor their symptoms while taking amphetamine.[66][67] Evidence from human studies indicates that therapeutic amphetamine use does not cause developmental abnormalities in the fetus or newborns (i.e., it is not a human teratogen), but amphetamine abuse does pose risks to the fetus.[67] Amphetamine has also been shown to pass into breast milk, so the IPCS and USFDA advise mothers to avoid breastfeeding when using it.[66][67] Due to the potential for reversible growth impairments,[note 8] the USFDA advises monitoring the height and weight of children and adolescents prescribed amphetamines.[66]
Side effects
The side effects of amphetamine are varied, and the amount of amphetamine used is the primary factor in determining the likelihood and severity of side effects.[22][31][35] Amphetamine products such as Adderall, Dexedrine, and their generic equivalents are currently approved by the USFDA for long-term therapeutic use.[29][31] Recreational use of amphetamine generally involves much larger doses, which have a greater risk of serious side effects than dosages used for therapeutic reasons.[35]
Physical
At normal therapeutic doses, the physical side effects of amphetamine vary widely by age and from person to person.[31] Cardiovascular side effects can include irregular heartbeat (usually an increased heart rate), hypertension (high blood pressure) or hypotension (low blood pressure) from a vasovagal response, and Raynaud's phenomenon (reduced blood flow to extremities).[31][35][68] Sexual side effects in males may include erectile dysfunction, frequent erections, or prolonged erections.[31] Abdominal side effects may include stomach pain, loss of appetite, nausea, and weight loss.[31] Other potential side effects include dry mouth, excessive grinding of the teeth, acne, profuse sweating, blurred vision, reduced seizure threshold, and tics (a type of movement disorder).[31][35][68] Dangerous physical side effects are rare at typical pharmaceutical doses.[35]
Amphetamine stimulates the medullary respiratory centers, producing faster and deeper breaths.[35] In a normal person at therapeutic doses, this effect is usually not noticeable, but when respiration is already compromised, it may be evident.[35] Amphetamine also induces contraction in the urinary bladder sphincter, the muscle which controls urination, which can result in difficulty urinating. This effect can be useful in treating bed wetting and loss of bladder control.[35] The effects of amphetamine on the gastrointestinal tract are unpredictable.[35] If intestinal activity is high, amphetamine may reduce gastrointestinal motility (the rate at which content moves through the digestive system);[35] however, amphetamine may increase motility when the smooth muscle of the tract is relaxed.[35] Amphetamine also has a slight analgesic effect and can enhance the pain relieving effects of opiates.[35]
USFDA commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of amphetamine or other ADHD stimulants.[sources 5]
Psychological
Common psychological effects of therapeutic doses can include increased alertness, apprehension, concentration, decreased sense of fatigue, mood swings (elated mood followed by mildly depressed mood), increased initiative, insomnia or wakefulness, self-confidence, and sociability.[31][35] Less common side effects include anxiety, change in libido, grandiosity, irritability, repetitive or obsessive behaviors, and restlessness;[sources 6] these effects depend on the user's personality and current mental state.[35] Amphetamine psychosis (e.g., delusions and paranoia) can occur in heavy users.[22][31][32] Although very rare, this psychosis can also occur at therapeutic doses during long-term therapy.[22][31][33] According to the USFDA, "there is no systematic evidence" that stimulants can produce aggressive behavior or hostility.[31]
Overdose
An amphetamine overdose can lead to many different symptoms, but is rarely fatal with appropriate care.[67][74] The severity of overdose symptoms vary positively with dosage and inversely with drug tolerance to amphetamine.[35][67] Tolerant individuals have been known to take as much as 5 grams of amphetamine, roughly 100 times the maximum daily therapeutic dose, in a day.[67] Symptoms of a moderate and extremely large overdose are listed below; fatal amphetamine poisoning usually also involves convulsions and coma.[22][35] Chronic overdose of amphetamine poses a high risk of developing an addiction, since high doses result in increased expression of the addiction gene ΔFosB.[75] Consistent aerobic exercise appears to magnitude-dependently reduce this risk.[76] Template:Amphetamine overdose
Addiction
This diagram depicts the signaling events in the brain's reward center that are induced by chronic high-dose exposure to psychostimulants that increase the concentration of synaptic dopamine, like amphetamine, methamphetamine, and phenethylamine. Following presynaptic dopamine and glutamate co-release by such psychostimulants,[77][78] postsynaptic receptors for these neurotransmitters trigger internal signaling events through a cAMP-dependent pathway and a calcium-dependent pathway that ultimately result in increased CREB phosphorylation.[77][75] Phosphorylated CREB increases levels of ΔFosB, which in turn represses the c-Fos gene with the help of corepressors;[77][79][80] c-Fos repression acts as a molecular switch that enables the accumulation of ΔFosB in the neuron.[81] A highly stable (phosphorylated) form of ΔFosB, one that persists in neurons for 1–2 months, slowly accumulates following repeated high-dose exposure to stimulants through this process.[79][80] ΔFosB functions as "one of the master control proteins" that produces addiction-related structural changes in the brain, and upon sufficient accumulation, with the help of its downstream targets (e.g., nuclear factor kappa B), it induces an addictive state.[79][80]
|
Addiction is a serious risk with heavy recreational amphetamine use, but is unlikely to arise from typical medical use at therapeutic doses.[22][34][35] Tolerance develops rapidly in amphetamine abuse, so periods of extended use require increasingly larger doses of the drug in order to achieve the same effect.[82][83]
Biomolecular mechanisms
Current models of addiction from chronic drug use involve alterations in gene expression in certain parts of the brain, particularly the nucleus accumbens.[84][85][86] The most important transcription factors[note 9] that produce these alterations are ΔFosB, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), and nuclear factor kappa B (NFκB).[85] ΔFosB is the most significant factor in drug addiction, since its overexpression in the nucleus accumbens is necessary and sufficient for many of the associated neural adaptations that occur;[85] it has been implicated in addictions to alcohol, cannabinoids, cocaine, nicotine, opiates, phenylcyclidine, and substituted amphetamines.[85][88][89] ΔJunD is the transcription factor which directly opposes ΔFosB.[85] Increases in nucleus accumbens ΔJunD expression using viral vectors can reduce or, with a large increase, even block many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[85] ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[85][88][90] Since natural rewards induce expression of ΔFosB just like drugs of abuse do, chronic acquisition of these rewards can result in a similar pathological state of addiction.[88][85] Consequently, ΔFosB is the key transcription factor involved in amphetamine addiction and amphetamine-induced sex addictions, a phenomenon known as dopamine dysregulation syndrome which has been observed in some patients taking dopaminergic medications.[88][90][91]
The effects of amphetamine on gene regulation are both dose- and route-dependent.[86] Most of the research on gene regulation and addiction is based upon animal studies with intravenous amphetamine administration at very high doses.[86] The few studies that have used equivalent (weight-adjusted) human therapeutic doses and oral administration show that these changes, if they occur, are relatively minor.[86]
Pharmacological treatments
A Cochrane Collaboration review on amphetamine and methamphetamine addiction and abuse indicates that the current evidence on effective treatments is extremely limited.[92] The review indicated that fluoxetine[note 10] and imipramine[note 11] have some limited benefits in treating abuse and addiction, but concluded that there is currently no effective pharmacological treatment for amphetamine addiction or abuse.[92] A corroborating review indicated that amphetamine addiction is mediated through increased activation of dopamine receptors and co-localized NMDA receptors in the mesolimbic dopamine pathway (a pathway in the brain that connects the ventral tegmental area to the nucleus accumbens).[93] This review also noted that magnesium ions and serotonin inhibit NMDA receptors and that the magnesium ions do so by blocking the receptor's calcium channels.[93] It also suggested that, based upon animal testing, pathological (addiction-inducing) amphetamine use significantly reduces the level of intracellular magnesium throughout the brain.[93] Supplemental magnesium,[note 12] like fluoxetine treatment, has been shown to reduce amphetamine self-administration (doses given to oneself) in both humans and lab animals.[92][93]
Behavioral treatments
Cognitive behavioral therapy is currently the most effective clinical treatment for psychostimulant addiction.[94] Additionally, research on the neurobiological effects of physical exercise suggests that consistent aerobic exercise, especially endurance exercise (e.g., marathon running), prevents the development of drug addiction and is an effective adjunct (supplemental) treatment for amphetamine addiction.[76][88] Exercise leads to better treatment outcomes when used as an adjunct treatment, particularly for psychostimulant addictions.[76] In particular, aerobic exercise decreases psychostimulant self-administration, reduces the reinstatement (i.e., relapse) of drug-seeking, and induces opposite effects on striatal dopamine receptor D2 (DRD2) signaling (increased DRD2 density) to those induced by pathological stimulant use (decreased DRD2 density).[88]
Withdrawal
According to another Cochrane Collaboration review on withdrawal in highly addicted amphetamine and methamphetamine abusers, "when chronic heavy users abruptly discontinue amphetamine use, many report a time-limited withdrawal syndrome that occurs within 24 hours of their last dose."[95] This review noted that withdrawal symptoms in chronic, high-dose users are frequent, occurring in up to 87.6% of cases, and persist for three to four weeks with a marked "crash" phase occurring during the first week.[95] Amphetamine withdrawal symptoms can include anxiety, drug craving, depressed mood, fatigue, increased appetite, increased movement or decreased movement, lack of motivation, sleeplessness or sleepiness, and lucid dreams.[95] The review indicated that withdrawal symptoms are associated with the degree of dependence, suggesting that therapeutic use would result in far milder discontinuation symptoms.[95] Manufacturer prescribing information does not indicate the presence of withdrawal symptoms following discontinuation of amphetamine use after an extended period at therapeutic doses.[96][97][98]
Psychosis
Abuse of amphetamine can result in a stimulant psychosis that may present with a variety of symptoms (e.g., paranoia and delusions).[32] A Cochrane Collaboration review on treatment for amphetamine, dextroamphetamine, and methamphetamine abuse-induced psychosis states that about 5–15% of users fail to recover completely.[32][99] According to the same review, there is at least one trial that shows antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[32] Psychosis very rarely arises from therapeutic use.[33][66]
Toxicity
In rodents and primates, sufficiently high doses of amphetamine cause dopaminergic neurotoxicity, or damage to dopamine neurons, which is characterized as reduced transporter and receptor function.[100] There is no evidence that amphetamine is directly neurotoxic in humans.[101][102] High-dose amphetamine can cause indirect neurotoxicity as a result of increased oxidative stress from reactive oxygen species and autoxidation of dopamine.[38][103][104]
Interactions
Many types of substances are known to interact with amphetamine, resulting in altered drug action or metabolism of amphetamine, the interacting substance, or both.[1][105] Inhibitors of the enzymes that metabolize amphetamine (i.e., CYP2D6 and flavin-containing monooxygenase 3) will prolong its elimination half-life.[5][105] Amphetamine also interacts with MAOIs, particularly monoamine oxidase A inhibitors, since both MAOIs and amphetamine increase plasma catecholamines; therefore, concurrent use of both is dangerous.[105] Amphetamine will modulate the activity of most psychoactive drugs. In particular, amphetamine may decrease the effects of sedatives and depressants and increase the effects of stimulants and antidepressants.[105] Amphetamine may also decrease the effects of antihypertensives and antipsychotics due to its effects on blood pressure and dopamine respectively.[105] In general, there is no significant interaction when consuming amphetamine with food, but the pH of gastrointestinal content and urine affects the absorption and excretion of amphetamine, respectively.[105] Acidic substances reduce the absorption of amphetamine and increase urinary excretion, and alkaline substances do the opposite.[105] Due to the effect pH has on absorption, amphetamine also interacts with gastric acid reducers such as proton pump inhibitors and H2 antihistamines, which increase gastrointestinal pH.[105]
Pharmacology
Pharmacodynamics
Pharmacodynamics of amphetamine in a dopamine neuron
Amphetamine enters the presynaptic neuron across the neuronal membrane or through DAT.[30] Once inside, it binds to TAAR1 or enters synaptic vesicles through VMAT2.[30][106] When amphetamine enters synaptic vesicles through VMAT2, it collapses the vesicular pH gradient, which in turn causes dopamine to be released into the cytosol (light tan-colored area) through VMAT2.[106][107] When amphetamine binds to TAAR1, it reduces the firing rate of the dopamine neuron via potassium channels and activates protein kinase A (PKA) and protein kinase C (PKC), which subsequently phosphorylate DAT.[30][108][109] PKA-phosphorylation causes DAT to withdraw into the presynaptic neuron (internalize) and cease transport.[30] PKC-phosphorylated DAT may either operate in reverse or, like PKA-phosphorylated DAT, internalize and cease transport.[30] Amphetamine is also known to increase intracellular calcium, an effect which is associated with DAT phosphorylation through a CAMKIIα-dependent pathway, in turn producing dopamine efflux.[110][111]
|
Amphetamine exerts its behavioral effects by altering the use of monoamines as neuronal signals in the brain, primarily in catecholamine neurons in the reward and executive function pathways of the brain, collectively known as the mesocorticolimbic projection.[30][47] The concentrations of the main neurotransmitters involved in reward circuitry and executive functioning, dopamine and norepinephrine, increase dramatically in a dose-dependent manner by amphetamine due to its effects on monoamine transporters.[30][47][106] The reinforcing and task saliency effects of amphetamine are mostly due to enhanced dopaminergic activity in the mesolimbic pathway.[24]
Amphetamine has been identified as a potent full agonist of trace amine-associated receptor 1 (TAAR1), a Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) discovered in 2001, which is important for regulation of brain monoamines.[30][112] Activation of TAAR1 increases cAMP production via adenylyl cyclase activation and inhibits monoamine transporter function.[30][113] Monoamine autoreceptors (e.g., D2 short, presynaptic α2, and presynaptic 5-HT1A) have the opposite effect of TAAR1, and together these receptors provide a regulatory system for monoamines.[30] Notably, amphetamine and trace amines bind to TAAR1, but not monoamine autoreceptors.[30] Imaging studies indicate that monoamine reuptake inhibition by amphetamine and trace amines is site specific and depends upon the presence of TAAR1 co-localization in the associated monoamine neurons.[30] As of 2010,[update] co-localization of TAAR1 and the dopamine transporter (DAT) has been visualized in rhesus monkeys, but co-localization of TAAR1 with the norepinephrine transporter (NET) and the serotonin transporter (SERT) has only been evidenced by messenger RNA (mRNA) expression.[30]
In addition to the neuronal monoamine transporters, amphetamine also inhibits vesicular monoamine transporter 2 (VMAT2), SLC1A1, SLC22A3, and SLC22A5.[sources 7] SLC1A1 is excitatory amino acid transporter 3 (EAAT3), a glutamate transporter located in neurons, SLC22A3 is an extraneuronal monoamine transporter that is present in astrocytes and SLC22A5 is a high-affinity carnitine transporter.[sources 7] Amphetamine is known to strongly induce cocaine- and amphetamine-regulated transcript (CART) gene expression,[118] a neuropeptide involved in feeding behavior, stress, and reward, which induces observable increases in neuronal development and survival in vitro.[119][120][121] The CART receptor has yet to be identified, but there is significant evidence that CART binds to a unique Gi/Go-coupled GPCR.[121][122] Amphetamine also inhibits monoamine oxidase at very high doses, resulting in less dopamine and phenethylamine metabolism and consequently higher concentrations of synaptic monoamines.[14][123] The full profile of amphetamine's short-term drug effects is derived through increased cellular communication or neurotransmission of dopamine,[30] serotonin,[30] norepinephrine,[30] epinephrine,[106] histamine,[106] CART peptides,[118] acetylcholine,[124][125] and glutamate,[126][127] which it effects through interactions with CART, EAAT3, TAAR1, and VMAT2.[sources 8]
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[128] Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.[35][128]
Dopamine
In certain brain regions, amphetamine increases the concentration of dopamine in the synaptic cleft.[30] Amphetamine can enter the presynaptic neuron either through DAT or by diffusing across the neuronal membrane directly.[30] As a consequence of DAT uptake, amphetamine produces competitive reuptake inhibition at the transporter.[30] Upon entering the presynaptic neuron, amphetamine activates TAAR1 which, through protein kinase A (PKA) and protein kinase C (PKC) signaling, causes DAT phosphorylation.[30] Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces reverse transporter function (dopamine efflux).[30][129] Amphetamine is also known to increase intracellular calcium, a known effect of TAAR1 activation, which is associated with DAT phosphorylation through a Ca2+/calmodulin-dependent protein kinase (CAMK)-dependent pathway, in turn producing dopamine efflux.[112][110][111] Through direct activation of G protein-coupled inwardly-rectifying potassium channels and increased dopamine release, TAAR1 reduces the firing rate of postsynaptic dopamine receptors, preventing a hyper-dopaminergic state.[130][108][109]
Amphetamine is also a substrate for the presynaptic vesicular monoamine transporter, VMAT2.[106] Following amphetamine uptake at VMAT2, the synaptic vesicle releases dopamine molecules into the cytosol in exchange.[106] Subsequently, the cytosolic dopamine molecules exit the presynaptic neuron via reverse transport at DAT.[30][106]
Norepinephrine
Similar to dopamine, amphetamine dose-dependently increases the level of synaptic norepinephrine, the direct precursor of epinephrine.[37][47] Based upon neuronal TAAR1 mRNA expression, amphetamine is thought to affect norepinephrine analogously to dopamine.[30][106][129] In other words, amphetamine induces TAAR1-mediated efflux and non-competitive reuptake inhibition at phosphorylated NET, competitive NET reuptake inhibition, and norepinephrine release from VMAT2.[30][106]
Serotonin
Amphetamine exerts analogous, yet less pronounced, effects on serotonin as on dopamine and norepinephrine.[30][47] Amphetamine affects serotonin via VMAT2 and, like norepinephrine, is thought to phosphorylate SERT via TAAR1.[30][106]
Other neurotransmitters
Amphetamine has no direct effect on acetylcholine neurotransmission, but several studies have noted that acetylcholine release increases after its use.[124][125] In lab animals, amphetamine increases acetylcholine levels in certain brain regions as a downstream effect.[124] In humans, a similar phenomenon occurs via the ghrelin-mediated cholinergic–dopaminergic reward link in the ventral tegmental area.[125] This heightened cholinergic activity leads to increased nicotinic receptor activation in the CNS, a factor which likely contributes to the nootropic effects of amphetamine.[131]
Extracellular levels of glutamate, the primary excitatory neurotransmitter in the brain, have been shown to increase upon exposure to amphetamine.[126][127] This cotransmission effect was found in the mesolimbic pathway, an area of the brain implicated in reward, where amphetamine is known to affect dopamine neurotransmission.[126][127] Amphetamine also induces effluxion of histamine from synaptic vesicles in CNS mast cells and histaminergic neurons through VMAT2.[106]
Pharmacokinetics
The oral bioavailability of amphetamine varies with gastrointestinal pH;[105] it is well absorbed from the gut, and bioavailability is typically over 75% for dextroamphetamine.[9] Amphetamine is a weak base with a pKa of 9–10;[1] consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[1][105] Conversely, an acidic pH means the drug is predominantly in a water soluble cationic (salt) form, and less is absorbed.[1] Approximately 15–40% of amphetamine circulating in the bloodstream is bound to plasma proteins.[10]
The half-life of amphetamine enantiomers differ and vary with urine pH.[1] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[1] An acidic diet will reduce the enantiomer half-lives to 8–11 hours; an alkaline diet will increase the range to 16–31 hours.[132][133] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[1] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[1] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[1] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[1] Amphetamine is usually eliminated within two days of the last oral dose.[132] Apparent half-life and duration of effect increase with repeated use and accumulation of the drug.[134]
The prodrug lisdexamfetamine is not as sensitive to pH as amphetamine when being absorbed in the gastrointestinal tract;[135] following absorption into the blood stream, it is converted by red blood cell-associated enzymes to dextroamphetamine via hydrolysis.[135] The elimination half-life of lisdexamfetamine is generally less than one hour.[135]
CYP2D6, dopamine β-hydroxylase, flavin-containing monooxygenase 3, butyrate-CoA ligase, and glycine N-acyltransferase are the enzymes known to metabolize amphetamine or its metabolites in humans.[sources 9] Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamfetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[1][132][136] Among these metabolites, the active sympathomimetics are 4‑hydroxyamphetamine,[137] 4‑hydroxynorephedrine,[138] and norephedrine.[139] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[1][132] The known pathways and detectable metabolites in humans include the following:[1][5][136] Template:Amphetamine Pharmacokinetics
Related endogenous compounds
Amphetamine has a very similar structure and function to the endogenous trace amines, which are naturally occurring neurotransmitter molecules produced in the human body and brain.[30][37] Among this group, the most closely related compounds are phenethylamine, the parent compound of amphetamine, and N-methylphenethylamine, an isomer of amphetamine (i.e., it has an identical molecular formula).[30][37][140] In humans, phenethylamine is produced directly from L-phenylalanine by the aromatic amino acid decarboxylase (AADC) enzyme, which converts L-DOPA into dopamine as well.[37][140] In turn, N‑methylphenethylamine is metabolized from phenethylamine by phenylethanolamine N-methyltransferase, the same enzyme that metabolizes norepinephrine into epinephrine.[37][140] Like amphetamine, both phenethylamine and N‑methylphenethylamine regulate monoamine neurotransmission via TAAR1;[30][140] unlike amphetamine, both of these substances are broken down by monoamine oxidase B, and therefore have a shorter half-life than amphetamine.[37][140]
Physical and chemical properties
Phenyl-2-nitropropene (right cups)
Amphetamine is a methyl homolog of the mammalian neurotransmitter phenethylamine with the chemical formula Template:Chemical formula. The carbon atom adjacent to the primary amine is a stereogenic center, and amphetamine is composed of a racemic 1:1 mixture of two enantiomeric mirror images.[15] This racemic mixture can be separated into its optical isomers:[note 13] levoamphetamine and dextroamphetamine.[15] Physically, at room temperature, the pure free base of amphetamine is a mobile, colorless, and volatile liquid with a characteristically strong amine odor, and acrid, burning taste.[141] Frequently prepared solid salts of amphetamine include amphetamine aspartate,[22] hydrochloride,[142] phosphate,[143] saccharate,[22] and sulfate,[22] the last of which is the most common amphetamine salt.[36] Amphetamine is also the parent compound of its own structural class, which includes a number of psychoactive derivatives.[15] In organic chemistry, amphetamine is an excellent chiral ligand for the stereoselective synthesis of 1,1'-bi-2-naphthol.[144]
Derivatives
Amphetamine derivatives, often referred to as "amphetamines" or "substituted amphetamines", are a broad range of chemicals that contain amphetamine as a "backbone".[145][146] The class includes stimulants like methamphetamine, serotonergic empathogens like MDMA (ecstasy), and decongestants like ephedrine, among other subgroups.[145][146] This class of chemicals is sometimes referred to collectively as the "amphetamine family."[147]
Synthesis
Template:Details3 Since the first preparation was reported in 1887,[148] numerous synthetic routes to amphetamine have been developed.[149][150] Many of these syntheses are based on classic organic reactions. One such example is the Friedel–Crafts alkylation of chlorobenzene by allyl chloride to yield beta chloropropylbenzene which is then reacted with ammonia to produce racemic amphetamine (method 1).[151] Another example employs the Ritter reaction (method 2). In this route, allylbenzene is reacted acetonitrile in sulfuric acid to yield an organosulfate which in turn is treated with sodium hydroxide to give amphetamine via an acetamide intermediate.[152][153] A third route starts with ethyl 3-oxobutanoate which through a double alkylation with methyl iodide followed by benzyl chloride can be converted into 2-methyl-3-phenyl-propanoic acid. This synthetic intermediate can be transformed into amphetamine using either a Hofmann or Curtius rearrangement (method 3).[154]
A significant number of amphetamine syntheses feature a reduction of a nitro, imine, oxime or other nitrogen-containing functional group.[149] In one such example, a Knoevenagel condensation of benzaldehyde with nitroethane yields phenyl-2-nitropropene. The double bond and nitro group of this intermediate is reduced using either catalytic hydrogenation or by treatment with lithium aluminium hydride (method 4).[155][156] Another method is the reaction of phenylacetone with ammonia, producing an imine intermediate that is reduced to the primary amine using hydrogen over a palladium catalyst or lithium aluminum hydride (method 5).[156]
The most common route of both legal and illicit amphetamine synthesis employs a non-metal reduction known as the Leuckart reaction (method 6).[36][156] In the first step, a reaction between phenylacetone and formamide, either using additional formic acid or formamide itself as a reducing agent, yields N-formylamphetamine. This intermediate is then hydrolyzed using hydrochloric acid, and subsequently basified, extracted with organic solvent, concentrated, and distilled to yield the free base. The free base is then dissolved in an organic solvent, sulfuric acid added, and amphetamine precipitates out as the sulfate salt.[156][157]
A number of chiral resolutions have been developed to separate the two enantiomers of amphetamine.[150] For example, racemic amphetamine can be treated with d-tartaric acid to form a diastereoisomeric salt which is fractionally crystallized to yield dextroamphetamine.[158] Chiral resolution remains the most economical method for obtaining optically pure amphetamine on a large scale.[159] In addition, several enantioselective syntheses of amphetamine have been developed. In one example, optically pure (R)-1-phenyl-ethanamine is condensed with phenylacetone to yield a chiral schiff base. In the key step, this intermediate is reduced by catalytic hydrogenation with a transfer of chirality to the carbon atom alpha to the amino group. Cleavage of the benzylic amine bond by hydrogenation yields optically pure dextroamphetamine.[159]
|
|
Detection in body fluids
Amphetamine is frequently measured in urine or blood as part of a drug test for sports, employment, poisoning diagnostics, and forensics.[sources 10] Techniques such as immunoassay, which is the most common form of amphetamine test, may cross-react with a number of sympathomimetic drugs.[163] Chromatographic methods specific for amphetamine are employed to prevent false positive results.[164] Chiral separation techniques may be employed to help distinguish the source of the drug, whether prescription amphetamine, prescription amphetamine prodrugs, (e.g., selegiline), over-the-counter drug products (e.g., Vicks VapoInhaler, which contains levomethamphetamine) or illicitly obtained substituted amphetamines.[164][165][166] Several prescription drugs produce amphetamine as a metabolite, including benzphetamine, clobenzorex, famprofazone, fenproporex, lisdexamfetamine, mesocarb, methamphetamine, prenylamine, and selegiline, among others.[27][167][168] These compounds may produce positive results for amphetamine on drug tests.[167][168] Amphetamine is generally only detectable by a standard drug test for approximately 24 hours, although a high dose may be detectable for two to four days.[163]
For the assays, a study noted that an enzyme multiplied immunoassay technique (EMIT) assay for amphetamine and methamphetamine may produce more false positives than liquid chromatography–tandem mass spectrometry.[165] Gas chromatography–mass spectrometry (GC–MS) of amphetamine and methamphetamine with the derivatizing agent (S)-(−)-trifluoroacetylprolyl chloride allows for the detection of methamphetamine in urine.[164] GC–MS of amphetamine and methamphetamine with the chiral derivatizing agent Mosher's acid chloride allows for the detection of both dextroamphetamine and dextromethamphetamine in urine.[164] Hence, the latter method may be used on samples that test positive using other methods to help distinguish between the various sources of the drug.[164]
History, society, and culture
| Substance | Mean estimate |
Low estimate |
High estimate |
|---|---|---|---|
| Cannabis | 177.63 | 125.30 | 227.27 |
| Cocaine | 17.24 | 13.99 | 20.92 |
| MDMA | 18.75 | 9.4 | 28.24 |
| Opiates | 16.37 | 12.80 | 20.23 |
| Opioids | 33.04 | 28.63 | 38.16 |
| Substituted amphetamines |
34.40 | 13.94 | 54.81 |
Amphetamine was first synthesized in 1887 in Germany by Romanian chemist Lazăr Edeleanu who named it phenylisopropylamine;[148][170][171] its stimulant effects remained unknown until 1927, when it was independently resynthesized by Gordon Alles and reported to have sympathomimetic properties.[171] Amphetamine had no pharmacological use until 1934, when Smith, Kline and French began selling it as an inhaler under the trade name Benzedrine as a decongestant.[28] During World War II, amphetamines and methamphetamine were used extensively by both the Allied and Axis forces for their stimulant and performance-enhancing effects.[148][172][173] As the addictive properties of the drug became known, governments began to place strict controls on the sale of amphetamine.[148] For example, during the early 1970s in the United States, amphetamine became a schedule II controlled substance under the Controlled Substances Act.[174] In spite of strict government controls, amphetamine has been used legally or illicitly by people from a variety of backgrounds, including authors,[175] musicians,[176] mathematicians,[177] and athletes.[23]
Amphetamine is still illegally synthesized today in clandestine labs and sold on the black market, primarily in European countries.[169] Among European Union (EU) member states, 1.2 million young adults used illicit amphetamine or methamphetamine in 2013.[178] During 2012, approximately 5.9 metric tons of illicit amphetamine were seized within EU member states;[178] the "street price" of illicit amphetamine within the EU ranged from €6–38 per gram during the same period.[178] Outside Europe, the illicit market for amphetamine is much smaller than the market for methamphetamine and MDMA.[169]
Legal status
As a result of the United Nations 1971 Convention on Psychotropic Substances, amphetamine became a schedule II controlled substance, as defined in the treaty, in all (183) state parties.[21] Consequently, it is heavily regulated in most countries.[179][180] Some countries, such as South Korea and Japan, have banned substituted amphetamines even for medical use.[181][182] In other nations, such as Canada (schedule I drug),[183] the United States (schedule II drug),[22] Thailand (category 1 narcotic),[184] and United Kingdom (class B drug),[185] amphetamine is in a restrictive national drug schedule that allows for its use as a medical treatment.[26][169]
Pharmaceutical products
The only currently prescribed amphetamine formulation that contains both enantiomers is Adderall.[note 3][15][27] Amphetamine is also prescribed in enantiopure and prodrug form as dextroamphetamine and lisdexamfetamine respectively.[29][186] Lisdexamfetamine is structurally different from amphetamine, and is inactive until it metabolizes into dextroamphetamine.[186] The free base of racemic amphetamine was previously available as Benzedrine, Psychedrine, and Sympatedrine.[15][27] Levoamphetamine was previously available as Cydril.[27] All current amphetamine pharmaceuticals are salts due to the comparatively high volatility of the free base.[27][29][36] Some of the current brands and their generic equivalents are listed below.
|
|
Notes
- ↑ Synonyms and alternate spellings include: 1-phenylpropan-2-amine (IUPAC name), α-methylbenzeneethanamine, α-methylphenethylamine, amfetamine (International Nonproprietary Name [INN]), β-phenylisopropylamine, desoxynorephedrine, and speed.[14][15][16]
- ↑ Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.[17]
Levoamphetamine and dextroamphetamine are also known as L-amph or levamfetamine (INN) and D-amph or dexamfetamine (INN) respectively.[14] - ↑ 3.0 3.1 "Adderall" is a brand name as opposed to a nonproprietary name; because the latter ("dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate, and amphetamine aspartate"[29]) is excessively long, this article exclusively refers to this amphetamine mixture by the brand name.
- ↑ Due to confusion that may arise from use of the plural form, this article will only use the terms "amphetamine" and "amphetamines" to refer to racemic amphetamine, levoamphetamine, and dextroamphetamine and reserve the term "substituted amphetamines" for the class.
- ↑ Again, due to confusion that may arise from use of the plural form, this article will only use "phenethylamine" and "phenethylamines" to refer to the compound itself and reserve the term "substituted phenethylamines" for the class.
- ↑ Cochrane Collaboration reviews are high quality meta-analytic systematic reviews of randomized controlled trials.[50]
- ↑ The statements supported by the USFDA come from prescribing information, which is the copyrighted intellectual property of the manufacturer and approved by the USFDA.
- ↑ In individuals who experience sub-normal height and weight gains, a rebound to normal levels is expected to occur if stimulant therapy is briefly interrupted.[44][45][68] The average reduction in final adult height from continuous stimulant therapy over a 3 year period is 2 cm.[68]
- ↑ Transcription factors are proteins that increase or decrease the expression of specific genes.[87]
- ↑ During short-term treatment, fluoxetine may decrease drug craving.[92]
- ↑ During "medium-term treatment," imipramine may extend the duration of adherence to addiction treatment.[92]
- ↑ The review indicated that magnesium L-aspartate and magnesium chloride produce significant changes in addictive behavior;[93] other forms of magnesium were not mentioned.
- ↑ Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.[17]
- Image legend
- ↑ (Text color) Transcription factors
Reference notes
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ↑ 2.0 2.1 Lemke TL, Williams DA, Roche VF, Zito W (2013). Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 648. ISBN 9781609133450.
Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ↑ 3.0 3.1 Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). J. Biol. Chem. 249 (2): 454–458. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ↑ 4.0 4.1 Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circ. Res. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ↑ 5.0 5.1 5.2 5.3 Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacol. Ther. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
- ↑ 6.0 6.1 Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". J. Pharmacol. Exp. Ther. 288 (3): 1251–1260. PMID 10027866.
- ↑ 7.0 7.1
- ↑ 8.0 8.1
- ↑ 9.0 9.1 "Pharmacology". Dextroamphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 5 November 2013.
- ↑ 10.0 10.1 "Pharmacology". Amphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 5 November 2013.
- ↑ 11.0 11.1 11.2 11.3 "Adderall IR Prescribing Information" (PDF). United States Food and Drug Administration. Barr Laboratories, Inc. March 2007. pp. 4–5. Retrieved 2 November 2013.
- ↑ "Properties: Predicted – EP|Suite". Amphetamine. Chemspider. Retrieved 6 November 2013.
- ↑ "Chemical and Physical Properties". Amphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 5 November 2013.
- ↑ 14.0 14.1 14.2 "Compound Summary". Amphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 13 October 2013.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 "Identification". Amphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 13 October 2013.
- ↑
- ↑ 17.0 17.1 "Enantiomer". IUPAC Goldbook. International Union of Pure and Applied Chemistry. doi:10.1351/goldbook.E02069. Archived from the original on 17 March 2013. Retrieved 14 March 2014.
One of a pair of molecular entities which are mirror images of each other and non-superposable.
- ↑ "Amphetamine". Medical Subject Headings. National Institutes of Health, National Library of Medicine. Retrieved 16 December 2013.
- ↑ "GUIDELINES ON THE USE OF INTERNATIONAL NONPROPRIETARY NAMES (INNs) FOR PHARMACEUTICAL SUBSTANCES". World Health Organization. 1997. Retrieved 1 December 2014.
In principle, INNs are selected only for the active part of the molecule which is usually the base, acid or alcohol. In some cases, however, the active molecules need to be expanded for various reasons, such as formulation purposes, bioavailability or absorption rate. In 1975 the experts designated for the selection of INN decided to adopt a new policy for naming such molecules. In future, names for different salts or esters of the same active substance should differ only with regard to the inactive moiety of the molecule. ... The latter are called modified INNs (INNMs).
- ↑ Yoshida T (1997). "Chapter 1: Use and Misuse of Amphetamines: An International Overview". In Klee H. Amphetamine Misuse: International Perspectives on Current Trends. Amsterdam, Netherlands: Harwood Academic Publishers. p. 2. ISBN 9789057020810. Retrieved 1 December 2014.
Amphetamine, in the singular form, properly applies to the racemate of 2-amino-1-phenylpropane. ... In its broadest context, however, the term can even embrace a large number of structurally and pharmacologically related substances.
- ↑ 21.0 21.1 "Convention on psychotropic substances". United Nations Treaty Collection. United Nations. Retrieved 11 November 2013.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. p. 11. Retrieved 30 December 2013.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Prim. Care. 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 24.8 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 318. ISBN 9780071481274.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in in normal subjects and those with ADHD. Positron emission tomography (PET) demonstrates that methylphenidate decreases regional cerebral blood flow in the doroslateral prefrontal cortex and posterior parietal cortex while improving performance of a spacial working memory task. This suggests that cortical networks that normally process spatial working memory become more efficient in response to the drug. ... [It] is now believed that dopamine and norepinephrine, but not serotonin, produce the beneficial effects of stimulants on working memory. At abused (relatively high) doses, stimulants can interfere with working memory and cognitive control ... stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks ... through indirect stimulation of dopamine and norepinephrine receptors.
- ↑ 25.0 25.1 25.2 Montgomery KA (June 2008). "Sexual desire disorders". Psychiatry (Edgmont). 5 (6): 50–55. PMC 2695750. PMID 19727285.
- ↑ 26.0 26.1 Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S (January 2008). "Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature". J. Am. Acad. Child Adolesc. Psychiatry. 47 (1): 21–31. doi:10.1097/chi.0b013e31815a56f1. PMID 18174822.
Stimulant misuse appears to occur both for performance enhancement and their euphorogenic effects, the latter being related to the intrinsic properties of the stimulants (e.g., IR versus ER profile) ...
Although useful in the treatment of ADHD, stimulants are controlled II substances with a history of preclinical and human studies showing potential abuse liability. - ↑ 27.00 27.01 27.02 27.03 27.04 27.05 27.06 27.07 27.08 27.09 27.10 27.11 Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ↑ 28.0 28.1 28.2 Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929–1950". J . Hist. Med. Allied Sci. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800.
- ↑ 29.0 29.1 29.2 29.3 29.4 29.5 29.6 29.7 29.8 "National Drug Code Amphetamine Search Results". National Drug Code Directory. United States Food and Drug Administration. Archived from the original on 7 February 2014. Retrieved 16 December 2013.
- ↑ 30.00 30.01 30.02 30.03 30.04 30.05 30.06 30.07 30.08 30.09 30.10 30.11 30.12 30.13 30.14 30.15 30.16 30.17 30.18 30.19 30.20 30.21 30.22 30.23 30.24 30.25 30.26 30.27 30.28 30.29 30.30 Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ↑ 31.00 31.01 31.02 31.03 31.04 31.05 31.06 31.07 31.08 31.09 31.10 31.11 31.12 31.13 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 4–8. Retrieved 30 December 2013.
- ↑ 32.0 32.1 32.2 32.3 32.4 Shoptaw SJ, Kao U, Ling W (2009). Shoptaw SJ, Ali R, ed. "Treatment for amphetamine psychosis". Cochrane Database Syst. Rev. (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMID 19160215.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - ↑ 33.0 33.1 33.2 Greydanus D. "Stimulant Misuse: Strategies to Manage a Growing Problem" (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Retrieved 2 November 2013.
- ↑ 34.0 34.1 Stolerman IP (2010). Stolerman IP, ed. Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. p. 78. ISBN 9783540686989.
- ↑ 35.00 35.01 35.02 35.03 35.04 35.05 35.06 35.07 35.08 35.09 35.10 35.11 35.12 35.13 35.14 35.15 35.16 35.17 35.18 35.19 35.20 Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York, USA: McGraw-Hill. ISBN 9780071624428.
- ↑ 36.0 36.1 36.2 36.3 "Amphetamine". European Monitoring Centre for Drugs and Drug Addiction. Retrieved 19 October 2013.
- ↑ 37.0 37.1 37.2 37.3 37.4 37.5 37.6 Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
Fig. 2. Synthetic and metabolic pathways for endogenous and exogenously administered trace amines and sympathomimetic amines ...
Trace amines are metabolized in the mammalian body via monoamine oxidase (MAO; EC 1.4.3.4) (Berry, 2004) (Fig. 2) ... It deaminates primary and secondary amines that are free in the neuronal cytoplasm but not those bound in storage vesicles of the sympathetic neurone ...
Thus, MAO inhibitors potentiate the peripheral effects of indirectly acting sympathomimetic amines ... this potentiation occurs irrespective of whether the amine is a substrate for MAO. An α-methyl group on the side chain, as in amphetamine and ephedrine, renders the amine immune to deamination so that they are not metabolized in the gut. Similarly, β-PEA would not be deaminated in the gut as it is a selective substrate for MAO-B which is not found in the gut ...
Brain levels of endogenous trace amines are several hundred-fold below those for the classical neurotransmitters noradrenaline, dopamine and serotonin but their rates of synthesis are equivalent to those of noradrenaline and dopamine and they have a very rapid turnover rate (Berry, 2004). Endogenous extracellular tissue levels of trace amines measured in the brain are in the low nanomolar range. These low concentrations arise because of their very short half-life ... - ↑ 38.0 38.1 Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L (August 2012). "Toxicity of amphetamines: an update". Arch. Toxicol. 86 (8): 1167–1231. doi:10.1007/s00204-012-0815-5. PMID 22392347.
- ↑ Berman S, O'Neill J, Fears S, Bartzokis G, London ED (2008). "Abuse of amphetamines and structural abnormalities in the brain". Ann. N. Y. Acad. Sci. 1141: 195–220. doi:10.1196/annals.1441.031. PMC 2769923. PMID 18991959.
- ↑ 40.0 40.1 Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- ↑ 41.0 41.1 Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". J. Clin. Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- ↑ 42.0 42.1 Frodl T, Skokauskas N (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects". Acta psychiatrica Scand. 125 (2): 114–126. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- ↑ 43.0 43.1 Millichap JG (2010). "Chapter 3: Medications for ADHD". In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 111–113. ISBN 9781441913968.
- ↑ 44.0 44.1 44.2 Huang YS, Tsai MH (July 2011). "Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge". CNS Drugs. 25 (7): 539–554. doi:10.2165/11589380-000000000-00000. PMID 21699268.
- ↑ 45.0 45.1 45.2 Millichap JG (2010). "Chapter 3: Medications for ADHD". In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 121–123, 125–127. ISBN 9781441913968.
- ↑ 46.0 46.1 46.2 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 154–157. ISBN 9780071481274.
- ↑ 47.0 47.1 47.2 47.3 47.4 Bidwell LC, McClernon FJ, Kollins SH (August 2011). "Cognitive enhancers for the treatment of ADHD". Pharmacol. Biochem. Behav. 99 (2): 262–274. doi:10.1016/j.pbb.2011.05.002. PMC 3353150. PMID 21596055.
- ↑ 48.0 48.1 "Stimulants for Attention Deficit Hyperactivity Disorder". WebMD. Healthwise. 12 April 2010. Retrieved 12 November 2013.
- ↑ Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S (February 2002). "Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults". J. Am. Acad. Child Adolesc. Psychiatry. 41 (2 Suppl): 26S–49S. doi:10.1097/00004583-200202001-00003. PMID 11833633.
- ↑ Scholten RJ, Clarke M, Hetherington J (August 2005). "The Cochrane Collaboration". Eur. J. Clin. Nutr. 59 Suppl 1: S147–S149, discussion S195–S196. doi:10.1038/sj.ejcn.1602188. PMID 16052183.
- ↑ Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M (2011). Castells X, ed. "Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults". Cochrane Database Syst. Rev. (6): CD007813. doi:10.1002/14651858.CD007813.pub2. PMID 21678370.
- ↑ Pringsheim T, Steeves T (April 2011). Pringsheim T, ed. "Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders". Cochrane Database Syst. Rev. (4): CD007990. doi:10.1002/14651858.CD007990.pub2. PMID 21491404.
- ↑ Martinsson L, Hårdemark H, Eksborg S (January 2007). Martinsson L, ed. "Amphetamines for improving recovery after stroke". Cochrane Database Syst. Rev. (1): CD002090. doi:10.1002/14651858.CD002090.pub2. PMID 17253474.
- ↑ Forsyth RJ, Jayamoni B, Paine TC (October 2006). Forsyth RJ, ed. "Monoaminergic agonists for acute traumatic brain injury". Cochrane Database Syst. Rev. (4): CD003984. doi:10.1002/14651858.CD003984.pub2. PMID 17054192.
- ↑ Harbeck-Seu A, Brunk I, Platz T, Vajkoczy P, Endres M, Spies C (April 2011). "A speedy recovery: amphetamines and other therapeutics that might impact the recovery from brain injury". Curr. Opin. Anaesthesiol. 24 (2): 144–153. doi:10.1097/ACO.0b013e328344587f. PMID 21386667.
- ↑ Devous MD, Trivedi MH, Rush AJ (April 2001). "Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers". J. Nucl. Med. 42 (4): 535–42. PMID 11337538.
- ↑ 57.0 57.1 57.2 Wood S, Sage JR, Shuman T, Anagnostaras SG (January 2014). "Psychostimulants and cognition: a continuum of behavioral and cognitive activation". Pharmacol. Rev. 66 (1): 193–221. doi:10.1124/pr.112.007054. PMID 24344115.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 266. ISBN 9780071481274.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ↑ Twohey M (26 March 2006). "Pills become an addictive study aid". JS Online. Archived from the original on 15 August 2007. Retrieved 2 December 2007.
- ↑ Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ (October 2006). "Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration". Pharmacotherapy. 26 (10): 1501–1510. doi:10.1592/phco.26.10.1501. PMC 1794223. PMID 16999660.
- ↑ Bracken NM (January 2012). "National Study of Substance Use Trends Among NCAA College Student-Athletes" (PDF). NCAA Publications. National Collegiate Athletic Association. Retrieved 8 October 2013.
- ↑ Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". Br. J. Pharmacol. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ↑ 63.0 63.1 63.2 63.3 Parr JW (July 2011). "Attention-deficit hyperactivity disorder and the athlete: new advances and understanding". Clin. Sports Med. 30 (3): 591–610. doi:10.1016/j.csm.2011.03.007. PMID 21658550.
- ↑ 64.0 64.1 64.2 Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R (May 2013). "Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing". Sports Med. 43 (5): 301–311. doi:10.1007/s40279-013-0030-4. PMID 23456493.
- ↑ Parker KL, Lamichhane D, Caetano MS, Narayanan NS (October 2013). "Executive dysfunction in Parkinson's disease and timing deficits". Front Integr Neurosci. 7: 75. doi:10.3389/fnint.2013.00075. PMC 3813949. PMID 24198770.
The neurotransmitter dopamine is released from projections originating in the midbrain. Manipulations of dopaminergic signaling profoundly influence interval timing, leading to the hypothesis that dopamine influences internal pacemaker, or “clock,” activity (Maricq and Church, 1983; Buhusi and Meck, 2005, 2009; Lake and Meck, 2013). For instance, amphetamine, which increases concentrations of dopamine at the synaptic cleft (Maricq and Church, 1983; Zetterström et al., 1983) advances the start of responding during interval timing (Taylor et al., 2007), whereas antagonists of D2 type dopamine receptors typically slow timing (Drew et al., 2003; Lake and Meck, 2013). ... Depletion of dopamine in healthy volunteers impairs timing (Coull et al., 2012), while amphetamine releases synaptic dopamine and speeds up timing (Taylor et al., 2007).
- ↑ 66.0 66.1 66.2 66.3 66.4 66.5 66.6 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 4–6. Retrieved 30 December 2013.
- ↑ 67.0 67.1 67.2 67.3 67.4 67.5 67.6 67.7 67.8 Heedes G; Ailakis J. "Amphetamine (PIM 934)". INCHEM. International Programme on Chemical Safety. Retrieved 24 June 2014.
- ↑ 68.0 68.1 68.2 68.3 Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child Adolesc. Psychiatr. Clin. N. Am. 17 (2): 459–474. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ↑ "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in children and young adults". United States Food and Drug Administration. 20 December 2011. Retrieved 4 November 2013.
- ↑ Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O'Duffy A, Connell FA, Ray WA (November 2011). "ADHD drugs and serious cardiovascular events in children and young adults". N. Engl. J. Med. 365 (20): 1896–1904. doi:10.1056/NEJMoa1110212. PMID 22043968.
- ↑ "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults". United States Food and Drug Administration. 15 December 2011. Retrieved 4 November 2013.
- ↑ Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV (December 2011). "ADHD medications and risk of serious cardiovascular events in young and middle-aged adults". JAMA. 306 (24): 2673–2683. doi:10.1001/jama.2011.1830. PMC 3350308. PMID 22161946.
- ↑ O'Connor PG (February 2012). "Amphetamines". Merck Manual for Health Care Professionals. Merck. Retrieved 8 May 2012.
- ↑ Spiller HA, Hays HL, Aleguas A (June 2013). "Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management". CNS Drugs. 27 (7): 531–543. doi:10.1007/s40263-013-0084-8. PMID 23757186.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- ↑ 75.0 75.1 Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
Most addictive drugs increase extracellular concentrations of dopamine (DA) in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), projection areas of mesocorticolimbic DA neurons and key components of the "brain reward circuit". Amphetamine achieves this elevation in extracellular levels of DA by promoting efflux from synaptic terminals. ... Chronic exposure to amphetamine induces a unique transcription factor delta FosB, which plays an essential role in long-term adaptive changes in the brain.
- ↑ 76.0 76.1 76.2 Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (September 2013). "Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis". Neurosci Biobehav Rev. 37 (8): 1622–44. doi:10.1016/j.neubiorev.2013.06.011. PMC 3788047. PMID 23806439.
these data show that exercise can affect dopaminergic signaling at many different levels, which may underlie its ability to modify vulnerability during drug use initiation. Exercise also produces neuroadaptations that may influence an individual's vulnerability to initiate drug use. Consistent with this idea, chronic moderate levels of forced treadmill running blocks not only subsequent methamphetamine-induced conditioned place preference, but also stimulant-induced increases in dopamine release in the NAc (Chen et al., 2008) and striatum (Marques et al., 2008). ... [These] findings indicate the efficacy of exercise at reducing drug intake in drug-dependent individuals ... wheel running [reduces] methamphetamine self-administration under extended access conditions (Engelmann et al., 2013) ... These findings suggest that exercise may "magnitude"-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuro-adaptive changes that develop during and following extended access to the drug. ... Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes (see Table 4). ... Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.
- ↑ 77.0 77.1 77.2 Renthal W, Nestler EJ (September 2009). "Chromatin regulation in drug addiction and depression". Dialogues Clin. Neurosci. 11 (3): 257–268. PMC 2834246. PMID 19877494.
[Psychostimulants] increase cAMP levels in striatum, which activates protein kinase A (PKA) and leads to phosphorylation of its targets. This includes the cAMP response element binding protein (CREB), the phosphorylation of which induces its association with the histone acetyltransferase, CREB binding protein (CBP) to acetylate histones and facilitate gene activation. This is known to occur on many genes including fosB and c-fos in response to psychostimulant exposure. ΔFosB is also upregulated by chronic psychostimulant treatments, and is known to activate certain genes (eg, cdk5) and repress others (eg, c-fos) where it recruits HDAC1 as a corepressor. ... Chronic exposure to psychostimulants increases glutamatergic [signaling] from the prefrontal cortex to the NAc. Glutamatergic signaling elevates Ca2+ levels in NAc postsynaptic elements where it activates CaMK (calcium/calmodulin protein kinases) signaling, which, in addition to phosphorylating CREB, also phosphorylates HDAC5.
Figure 2: Psychostimulant-induced signaling events - ↑ Broussard JI (January 2012). "Co-transmission of dopamine and glutamate". J. Gen. Physiol. 139 (1): 93–96. doi:10.1085/jgp.201110659. PMC 3250102. PMID 22200950.
Coincident and convergent input often induces plasticity on a postsynaptic neuron. The NAc integrates processed information about the environment from basolateral amygdala, hippocampus, and prefrontal cortex (PFC), as well as projections from midbrain dopamine neurons. Previous studies have demonstrated how dopamine modulates this integrative process. For example, high frequency stimulation potentiates hippocampal inputs to the NAc while simultaneously depressing PFC synapses (Goto and Grace, 2005). The converse was also shown to be true; stimulation at PFC potentiates PFC–NAc synapses but depresses hippocampal–NAc synapses. In light of the new functional evidence of midbrain dopamine/glutamate co-transmission (references above), new experiments of NAc function will have to test whether midbrain glutamatergic inputs bias or filter either limbic or cortical inputs to guide goal-directed behavior.
- ↑ 79.0 79.1 79.2 Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB serves as one of the master control proteins governing this structural plasticity. ... ΔFosB also represses G9a expression, leading to reduced repressive histone methylation at the cdk5 gene. The net result is gene activation and increased CDK5 expression. ... In contrast, ΔFosB binds to the c-fos gene and recruits several co-repressors, including HDAC1 (histone deacetylase 1) and SIRT 1 (sirtuin 1). ... The net result is c-fos gene repression.
Figure 4: Epigenetic basis of drug regulation of gene expression - ↑ 80.0 80.1 80.2 Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clin. Psychopharmacol. Neurosci. 10 (3): 136–143. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
The 35-37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB ... In contrast, the ability of ΔFosB to repress the c-Fos gene occurs in concert with the recruitment of a histone deacetylase and presumably several other repressive proteins such as a repressive histone methyltransferase
- ↑ Nestler EJ (October 2008). "Review. Transcriptional mechanisms of addiction: role of DeltaFosB". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 363 (1507): 3245–3255. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924.
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure
- ↑ "Amphetamines: Drug Use and Abuse". Merck Manual Home Edition. Merck. February 2003. Archived from the original on 17 February 2007. Retrieved 28 February 2007.
- ↑ Pérez-Mañá C, Castells X, Torrens M, Capellà D, Farre M (2013). Pérez-Mañá C, ed. "Efficacy of psychostimulant drugs for amphetamine abuse or dependence". Cochrane Database Syst. Rev. 9: CD009695. doi:10.1002/14651858.CD009695.pub2. PMID 23996457.
- ↑ Hyman SE, Malenka RC, Nestler EJ (2006). "Neural mechanisms of addiction: the role of reward-related learning and memory". Annu. Rev. Neurosci. 29: 565–598. doi:10.1146/annurev.neuro.29.051605.113009. PMID 16776597.
- ↑ 85.0 85.1 85.2 85.3 85.4 85.5 85.6 85.7 Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states.
- ↑ 86.0 86.1 86.2 86.3 Steiner H, Van Waes V (January 2013). "Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants". Prog. Neurobiol. 100: 60–80. doi:10.1016/j.pneurobio.2012.10.001. PMC 3525776. PMID 23085425.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 4: Signal Transduction in the Brain". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 94. ISBN 9780071481274.
All living cells depend on the regulation of gene expression by extracellular signals for their development, homeostasis, and adaptation to the environment. Indeed, many signal transduction pathways function primarily to modify transcription factors that alter the expression of specific genes. Thus, neurotransmitters, growth factors, and drugs change patterns of gene expression in cells and in turn affect many aspects of nervous system functioning, including the formation of long-term memories. Many drugs that require prolonged administration, such as antidepressants and antipsychotics, trigger changes in gene expression that are thought to be therapeutic adaptations to the initial action of the drug.
- ↑ 88.0 88.1 88.2 88.3 88.4 88.5 Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–1122. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101.
Similar to environmental enrichment, studies have found that exercise reduces self-administration and relapse to drugs of abuse (Cosgrove et al., 2002; Zlebnik et al., 2010). There is also some evidence that these preclinical findings translate to human populations, as exercise reduces withdrawal symptoms and relapse in abstinent smokers (Daniel et al., 2006; Prochaska et al., 2008), and one drug recovery program has seen success in participants that train for and compete in a marathon as part of the program (Butler, 2005). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008).
- ↑ Kanehisa Laboratories (29 October 2014). "Alcoholism – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ↑ 90.0 90.1 Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M (2012). "Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms". J. Psychoactive Drugs. 44 (1): 38–55. doi:10.1080/02791072.2012.662112. PMC 4040958. PMID 22641964.
It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. ... these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry.
- ↑ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). "Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator". J. Neurosci. 33 (8): 3434–3442. doi:10.1523/JNEUROSCI.4881-12.2013. PMC 3865508. PMID 23426671.
Drugs of abuse induce neuroplasticity in the natural reward pathway, specifically the nucleus accumbens (NAc), thereby causing development and expression of addictive behavior. ... Together, these findings demonstrate that drugs of abuse and natural reward behaviors act on common molecular and cellular mechanisms of plasticity that control vulnerability to drug addiction, and that this increased vulnerability is mediated by ΔFosB and its downstream transcriptional targets. ... Sexual behavior is highly rewarding (Tenk et al., 2009), and sexual experience causes sensitized drug-related behaviors, including cross-sensitization to amphetamine (Amph)-induced locomotor activity (Bradley and Meisel, 2001; Pitchers et al., 2010a) and enhanced Amph reward (Pitchers et al., 2010a). Moreover, sexual experience induces neural plasticity in the NAc similar to that induced by psychostimulant exposure, including increased dendritic spine density (Meisel and Mullins, 2006; Pitchers et al., 2010a), altered glutamate receptor trafficking, and decreased synaptic strength in prefrontal cortex-responding NAc shell neurons (Pitchers et al., 2012). Finally, periods of abstinence from sexual experience were found to be critical for enhanced Amph reward, NAc spinogenesis (Pitchers et al., 2010a), and glutamate receptor trafficking (Pitchers et al., 2012). These findings suggest that natural and drug reward experiences share common mechanisms of neural plasticity
- ↑ 92.0 92.1 92.2 92.3 92.4 Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P (2001). Srisurapanont M, ed. "Treatment for amphetamine dependence and abuse". Cochrane Database Syst. Rev. (4): CD003022. doi:10.1002/14651858.CD003022. PMID 11687171.
Although there are a variety of amphetamines and amphetamine derivatives, the word "amphetamines" in this review stands for amphetamine, dextroamphetamine and methamphetamine only.
- ↑ 93.0 93.1 93.2 93.3 93.4 Nechifor M (March 2008). "Magnesium in drug dependences". Magnes. Res. 21 (1): 5–15. PMID 18557129.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 386. ISBN 9780071481274.
Currently, cognitive–behavioral therapies are the most successful treatment available for preventing the relapse of psychostimulant use.
- ↑ 95.0 95.1 95.2 95.3 Shoptaw SJ, Kao U, Heinzerling K, Ling W (2009). Shoptaw SJ, ed. "Treatment for amphetamine withdrawal". Cochrane Database Syst. Rev. (2): CD003021. doi:10.1002/14651858.CD003021.pub2. PMID 19370579.
The prevalence of this withdrawal syndrome is extremely common (Cantwell 1998; Gossop 1982) with 87.6% of 647 individuals with amphetamine dependence reporting six or more signs of amphetamine withdrawal listed in the DSM when the drug is not available (Schuckit 1999) ... The severity of withdrawal symptoms is greater in amphetamine dependent individuals who are older and who have more extensive amphetamine use disorders (McGregor 2005). Withdrawal symptoms typically present within 24 hours of the last use of amphetamine, with a withdrawal syndrome involving two general phases that can last 3 weeks or more. The first phase of this syndrome is the initial "crash" that resolves within about a week (Gossop 1982;McGregor 2005) ...
- ↑ "Adderall IR Prescribing Information" (PDF). United States Food and Drug Administration. Barr Laboratories, Inc. March 2007. Retrieved 4 November 2013.
- ↑ "Dexedrine Medication Guide" (PDF). United States Food and Drug Administration. Amedra Pharmaceuticals LLC. May 2013. Retrieved 4 November 2013.
- ↑ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Retrieved 30 December 2013.
- ↑ Hofmann FG (1983). A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects (2nd ed.). New York, USA: Oxford University Press. p. 329. ISBN 9780195030570.
- ↑ Advokat C (2007). "Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD". J. Atten. Disord. 11 (1): 8–16. doi:10.1177/1087054706295605. PMID 17606768.
- ↑ "Amphetamine". Hazardous Substances Data Bank. National Library of Medicine. Retrieved 26 February 2014.
Direct toxic damage to vessels seems unlikely because of the dilution that occurs before the drug reaches the cerebral circulation.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and addictive disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- ↑ Sulzer D, Zecca L (February 2000). "Intraneuronal dopamine-quinone synthesis: a review". Neurotox. Res. 1 (3): 181–195. doi:10.1007/BF03033289. PMID 12835101.
- ↑ Miyazaki I, Asanuma M (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself". Acta Med. Okayama. 62 (3): 141–150. PMID 18596830.
- ↑ 105.0 105.1 105.2 105.3 105.4 105.5 105.6 105.7 105.8 105.9 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 8–10. Retrieved 30 December 2013.
- ↑ 106.00 106.01 106.02 106.03 106.04 106.05 106.06 106.07 106.08 106.09 106.10 106.11 106.12 106.13 Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216: 86–98. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).
- ↑
- ↑ 108.0 108.1 Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front Syst Neurosci. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ↑ 109.0 109.1 mct (28 January 2012). "TAAR1". GenAtlas. University of Paris. Retrieved 29 May 2014.
• tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA) - ↑ 110.0 110.1 110.2 Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (July 2014). "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron. 83 (2): 404–416. doi:10.1016/j.neuron.2014.05.043. PMC 4159050. PMID 25033183.
AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012).
- ↑ 111.0 111.1 Vaughan RA, Foster JD (September 2013). "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].
- ↑ 112.0 112.1 Maguire JJ, Davenport AP (2 December 2014). "TA1 receptor". IUPHAR database. International Union of Basic and Clinical Pharmacology. Retrieved 8 December 2014.
Comments: Tyramine causes an increase in intracellular cAMP in HEK293 or COS-7 cells expressing the TA1 receptor in vitro [4,6,18]. In addition, coupling to a promiscuous Gαq has been observed, resulting in increased intracellular calcium concentration [24].
- ↑ Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C (July 2001). "Trace amines: identification of a family of mammalian G protein-coupled receptors". Proc. Natl. Acad. Sci. U.S.A. 98 (16): 8966–8971. doi:10.1073/pnas.151105198. PMC 55357. PMID 11459929.
- ↑ 114.0 114.1 "SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 [ Homo sapiens (human) ]". NCBI Gene. National Center for Biotechnology Information. Retrieved 11 November 2014.
Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. ... internalization of EAAT3 triggered by amphetamine increases glutamatergic signaling and thus contributes to the effects of amphetamine on neurotransmission.
- ↑ Zhu HJ, Appel DI, Gründemann D, Markowitz JS (July 2010). "Interaction of organic cation transporter 3 (SLC22A3) and amphetamine". J. Neurochem. 114 (1): 142–149. doi:10.1111/j.1471-4159.2010.06738.x. PMC 3775896. PMID 20402963.
- ↑ Rytting E, Audus KL (January 2005). "Novel organic cation transporter 2-mediated carnitine uptake in placental choriocarcinoma (BeWo) cells". J. Pharmacol. Exp. Ther. 312 (1): 192–198. doi:10.1124/jpet.104.072363. PMID 15316089.
- ↑ Inazu M, Takeda H, Matsumiya T (August 2003). "[The role of glial monoamine transporters in the central nervous system]". Nihon Shinkei Seishin Yakurigaku Zasshi (in Japanese). 23 (4): 171–178. PMID 13677912.
- ↑ 118.0 118.1 118.2 Vicentic A, Jones DC (February 2007). "The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction". J. Pharmacol. Exp. Ther. 320 (2): 499–506. doi:10.1124/jpet.105.091512. PMID 16840648.
The physiological importance of CART was further substantiated in numerous human studies demonstrating a role of CART in both feeding and psychostimulant addiction. ... Colocalization studies also support a role for CART in the actions of psychostimulants. ... CART and DA receptor transcripts colocalize (Beaudry et al., 2004). Second, dopaminergic nerve terminals in the NAc synapse on CART-containing neurons (Koylu et al., 1999), hence providing the proximity required for neurotransmitter signaling. These studies suggest that DA plays a role in regulating CART gene expression possibly via the activation of CREB. Indeed, CART gene expression is regulated via cAMP signaling and pCREB in vivo as centrally administered forskolin activated cAMP, phosphorylated CREB, and increased CART mRNA and peptide levels (Jones and Kuhar, 2006).
- ↑ Zhang M, Han L, Xu Y (June 2012). "Roles of cocaine- and amphetamine-regulated transcript in the central nervous system". Clin. Exp. Pharmacol. Physiol. 39 (6): 586–592. doi:10.1111/j.1440-1681.2011.05642.x. PMID 22077697.
Numerous studies have established the role of CART in food intake, maintenance of bodyweight, stress control, reward and pain transmission. Recently, it was demonstrated that CART, as a neurotrophic peptide, had a cerebroprotective against focal ischaemic stroke and inhibited the neurotoxicity of β-amyloid protein, which focused attention on the role of CART in the central nervous system (CNS) and neurological diseases. 3. In fact, little is known about the way in which CART peptide interacts with its receptors, initiates downstream cascades and finally exerts its neuroprotective effect under normal or pathological conditions. The literature indicates that there are many factors, such as regulation of the immunological system and protection against energy failure, that may be involved in the cerebroprotection afforded by CART
- ↑ "Biomolecular Interactions and Pathways". Amphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 13 October 2013.
- ↑ 121.0 121.1 Vicentic A, Lakatos A, Jones D (August 2006). "The CART receptors: background and recent advances". Peptides. 27 (8): 1934–1937. doi:10.1016/j.peptides.2006.03.031. PMID 16713658.
- ↑ Lin Y, Hall RA, Kuhar MJ (October 2011). "CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6–38 as a CART receptor antagonist". Neuropeptides. 45 (5): 351–358. doi:10.1016/j.npep.2011.07.006. PMC 3170513. PMID 21855138.
- ↑ "Monoamine oxidase (Homo sapiens)". BRENDA. Technische Universität Braunschweig. 1 January 2014. Retrieved 4 May 2014.
- ↑ 124.0 124.1 124.2 Hutson PH, Tarazi FI, Madhoo M, Slawecki C, Patkar AA (September 2014). "Preclinical pharmacology of amphetamine: implications for the treatment of neuropsychiatric disorders". Pharmacol. Ther. 143 (3): 253–264. doi:10.1016/j.pharmthera.2014.03.005. PMID 24657455.
- ↑ 125.0 125.1 125.2 Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (June 2011). "The role of the central ghrelin system in reward from food and chemical drugs". Mol. Cell. Endocrinol. 340 (1): 80–87. doi:10.1016/j.mce.2011.02.017. PMID 21354264.
- ↑ 126.0 126.1 126.2 Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A (June 2010). "Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate". J. Neurosci. 30 (24): 8229–8233. doi:10.1523/JNEUROSCI.1754-10.2010. PMC 2918390. PMID 20554874.
- ↑ 127.0 127.1 127.2 Gu XL (October 2010). "Deciphering the corelease of glutamate from dopaminergic terminals derived from the ventral tegmental area". J. Neurosci. 30 (41): 13549–13551. doi:10.1523/JNEUROSCI.3802-10.2010. PMC 2974325. PMID 20943895.
- ↑ 128.0 128.1 Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ↑ 129.0 129.1 Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (March 2009). "International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature". Pharmacol. Rev. 61 (1): 1–8. doi:10.1124/pr.109.001107. PMC 2830119. PMID 19325074.
- ↑ Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC (May 2011). "TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity". Proc. Natl. Acad. Sci. U.S.A. 108 (20): 8485–8490. doi:10.1073/pnas.1103029108. PMC 3101002. PMID 21525407.
- ↑ Levin ED, Bushnell PJ, Rezvani AH (August 2011). "Attention-modulating effects of cognitive enhancers". Pharmacol. Biochem. Behav. 99 (2): 146–154. doi:10.1016/j.pbb.2011.02.008. PMC 3114188. PMID 21334367.
- ↑ 132.0 132.1 132.2 132.3 "Biomedical Effects and Toxicity". Amphetamine. Pubchem Compound. National Center for Biotechnology Information. Retrieved 12 October 2013.
- ↑ "Biological Half-Life". AMPHETAMINE. United States National Library of Medicine – Toxnet. Hazardous Substances Data Bank. Retrieved 5 January 2014.
Concentrations of (14)C-amphetamine declined less rapidly in the plasma of human subjects maintained on an alkaline diet (urinary pH > 7.5) than those on an acid diet (urinary pH < 6). Plasma half-lives of amphetamine ranged between 16-31 hr & 8-11 hr, respectively, & the excretion of (14)C in 24 hr urine was 45 & 70%.
- ↑ Richard RA (1999). "Route of Administration". Chapter 5—Medical Aspects of Stimulant Use Disorders. National Center for Biotechnology Information Bookshelf. Treatment Improvement Protocol 33. Substance Abuse and Mental Health Services Administration.
- ↑ 135.0 135.1 135.2 "Vyvanse Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 25 February 2013.
- ↑ 136.0 136.1 Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". J. Pharm. Biomed. Anal. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ↑ "Compound Summary". p-Hydroxyamphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 15 October 2013.
- ↑ "Compound Summary". p-Hydroxynorephedrine. PubChem Compound. National Center for Biotechnology Information. Retrieved 15 October 2013.
- ↑ "Compound Summary". Phenylpropanolamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 15 October 2013.
- ↑ 140.0 140.1 140.2 140.3 140.4 Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
In addition to the main metabolic pathway, TAs can also be converted by nonspecific N-methyltransferase (NMT) [22] and phenylethanolamine N-methyltransferase (PNMT) [23] to the corresponding secondary amines (e.g. synephrine [14], N-methylphenylethylamine and N-methyltyramine [15]), which display similar activities on TAAR1 (TA1) as their primary amine precursors.
- ↑ "Chemical and Physical Properties". Amphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 13 October 2013.
- ↑ "Amphetamine Hydrochloride". Pubchem Compound. National Center for Biotechnology Information. Retrieved 8 November 2013.
- ↑ "Amphetamine Phosphate". Pubchem Compound. National Center for Biotechnology Information. Retrieved 8 November 2013.
- ↑ Brussee J, Jansen ACA (1983). "A highly stereoselective synthesis of s(-)-[1,1'-binaphthalene]-2,2'-diol". Tetrahedron Lett. 24 (31): 3261–3262. doi:10.1016/S0040-4039(00)88151-4.
- ↑ 145.0 145.1 "Compound Summary". Amphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 30 September 2013.
- ↑ 146.0 146.1 Schep LJ, Slaughter RJ, Beasley DM (August 2010). "The clinical toxicology of metamfetamine". Clin. Toxicol. (Phila.). 48 (7): 675–694. doi:10.3109/15563650.2010.516752. ISSN 1556-3650. PMID 20849327.
- ↑ "Amphetamine, Methamphetamine, & Cystal Meth". Addiction Prevention Centre. Retrieved 10 October 2013.
- ↑ 148.0 148.1 148.2 148.3 "Historical overview of methamphetamine". Vermont Department of Health. Government of Vermont. Retrieved 29 January 2012.
- ↑ 149.0 149.1 Allen A, Cantrell TS (August 1989). "Synthetic reductions in clandestine amphetamine and methamphetamine laboratories: A review". Forensic Science International. 42 (3): 183–199. doi:10.1016/0379-0738(89)90086-8.
- ↑ 150.0 150.1 Allen A, Ely R (2009). "Review: Synthetic Methods for Amphetamine" (PDF). Crime Scene. Northwest Association of Forensic Scientists. 37 (2): 15–25. Retrieved 6 December 2014.
- ↑ Patrick TM, McBee ET, Hass HB (June 1946). "Synthesis of arylpropylamines; from allyl chloride". J. Am. Chem. Soc. 68: 1009–1011. doi:10.1021/ja01210a032. PMID 20985610.
- ↑ Ritter JJ, Kalish J (December 1948). "A new reaction of nitriles; synthesis of t-carbinamines". J. Am. Chem. Soc. 70 (12): 4048–4050. doi:10.1021/ja01192a023. PMID 18105933.
- ↑ Krimen LI, Cota DJ (1969). Organic Reactions. 17: 216. doi:10.1002/0471264180.or017.03. Missing or empty
|title=(help) - ↑ US patent 2413493, Bitler WP, Flisik AC, Leonard N, "Synthesis of isomer-free benzyl methyl acetoacetic methyl ester", published 31 December 1946, assigned to Kay Fries Chemicals Inc
- ↑ Collins M, Salouros H, Cawley AT, Robertson J, Heagney AC, Arenas-Queralt A (June 2010). "δ13C and δ2H isotope ratios in amphetamine synthesized from benzaldehyde and nitroethane". Rapid Commun. Mass Spectrom. 24 (11): 1653–1658. doi:10.1002/rcm.4563. PMID 20486262.
- ↑ 156.0 156.1 156.2 156.3 "Recommended methods of the identification and analysis of amphetamine, methamphetamine, and their ring-substituted analogues in seized materials" (PDF). United Nations Office on Drugs and Crime. United Nations. 2006. pp. 9–12. Retrieved 14 October 2013.
- ↑ Pollard CB, Young DC (May 1951). "The Mechanism of the Leuckart Reaction". J. Org. Chem. 16 (5): 661–672. doi:10.1021/jo01145a001.
- ↑ US patent 2276508, Nabenhauer FP, "Method for the separation of optically active alpha-methylphenethylamine", published 17 March 1942, assigned to Smith Kline French
- ↑ 159.0 159.1 Gray DL (2007). "Approved Treatments for Attention Deficit Hyperactivity Disorder: Amphetamine (Adderall), Methylphenidate (Ritalin), and Atomoxetine (Straterra)". In Johnson DS, Li JJ. The Art of Drug Synthesis. New York, USA: Wiley-Interscience. p. 247. ISBN 9780471752158.
- ↑ Kraemer T, Maurer HH (August 1998). "Determination of amphetamine, methamphetamine and amphetamine-derived designer drugs or medicaments in blood and urine". J. Chromatogr. B Biomed. Sci. Appl. 713 (1): 163–187. doi:10.1016/S0378-4347(97)00515-X. PMID 9700558.
- ↑ Kraemer T, Paul LD (August 2007). "Bioanalytical procedures for determination of drugs of abuse in blood". Anal. Bioanal. Chem. 388 (7): 1415–1435. doi:10.1007/s00216-007-1271-6. PMID 17468860.
- ↑ Goldberger BA, Cone EJ (July 1994). "Confirmatory tests for drugs in the workplace by gas chromatography-mass spectrometry". J. Chromatogr. A. 674 (1–2): 73–86. doi:10.1016/0021-9673(94)85218-9. PMID 8075776.
- ↑ 163.0 163.1 "Clinical Drug Testing in Primary Care" (PDF). Substance Abuse and Mental Health Services Administration. Technical Assistance Publication Series 32. United States Department of Health and Human Services. 2012. Retrieved 31 October 2013.
- ↑ 164.0 164.1 164.2 164.3 164.4 Paul BD, Jemionek J, Lesser D, Jacobs A, Searles DA (September 2004). "Enantiomeric separation and quantitation of (±)-amphetamine, (±)-methamphetamine, (±)-MDA, (±)-MDMA, and (±)-MDEA in urine specimens by GC-EI-MS after derivatization with (R)-(-)- or (S)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (MTPA)". J. Anal. Toxicol. 28 (6): 449–455. doi:10.1093/jat/28.6.449. PMID 15516295.
- ↑ 165.0 165.1 Verstraete AG, Heyden FV (2005). "Comparison of the sensitivity and specificity of six immunoassays for the detection of amphetamines in urine". J. Anal. Toxicol. 29 (5): 359–364. doi:10.1093/jat/29.5.359. PMID 16105261.
- ↑ Baselt RC (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, USA: Biomedical Publications. pp. 85–88. ISBN 9780962652387.
- ↑ 167.0 167.1 Musshoff F (February 2000). "Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine". Drug Metab. Rev. 32 (1): 15–44. doi:10.1081/DMR-100100562. PMID 10711406.
- ↑ 168.0 168.1 Cody JT (May 2002). "Precursor medications as a source of methamphetamine and/or amphetamine positive drug testing results". J. Occup. Environ. Med. 44 (5): 435–450. doi:10.1097/00043764-200205000-00012. PMID 12024689.
- ↑ 169.0 169.1 169.2 169.3 Mohan J, ed. (June 2014). "World Drug Report 2014" (PDF). United Nations Office on Drugs and Crime. pp. 3, 123–152. Retrieved 18 August 2014.
- ↑ Rassool GH (2009). Alcohol and Drug Misuse: A Handbook for Students and Health Professionals. London, England: Routledge. p. 113. ISBN 9780203871171.
- ↑ 171.0 171.1 Sulzer D, Sonders MS, Poulsen NW, Galli A (April 2005). "Mechanisms of neurotransmitter release by amphetamines: a review". Prog. Neurobiol. 75 (6): 406–433. doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
- ↑ Rasmussen N (2011). "Medical science and the military: the Allies' use of amphetamine during World War II". J. Interdiscip. Hist. 42 (2): 205–233. doi:10.1162/JINH_a_00212. PMID 22073434.
- ↑ Defalque RJ, Wright AJ (April 2011). "Methamphetamine for Hitler's Germany: 1937 to 1945". Bull. Anesth. Hist. 29 (2): 21–24, 32. PMID 22849208.
- ↑ "Controlled Substances Act". United States Food and Drug Administration. 11 June 2009. Retrieved 4 November 2013.
- ↑ Gyenis A. "Forty Years of On the Road 1957–1997". wordsareimportant.com. DHARMA beat. Archived from the original on 14 February 2008. Retrieved 18 March 2008.
- ↑ Wilson A (2008). "Mixing the Medicine: The unintended consequence of amphetamine control on the Northern Soul Scene" (PDF). Internet Journal of Criminology. Retrieved 25 May 2013.
- ↑ Hill J (4 June 2004). "Paul Erdos, Mathematical Genius, Human (In That Order)" (PDF). untruth.org. Retrieved 2 November 2013.
- ↑ 178.0 178.1 178.2 "European drug report 2014: Trends and developments" (PDF). Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction. May 2014: 13, 24. doi:10.2810/32306. ISSN 2314-9086. Retrieved 18 August 2014.
1.2 million or 0.9% of young adults (15–34) used amphetamines in the last year
- ↑ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide (PDF). New York, USA: United Nations. ISBN 9789211482232. Retrieved 11 November 2013.
- ↑ "List of psychotropic substances under international control" (PDF). International Narcotics Control Board. United Nations. August 2003. Archived from the original (PDF) on 5 December 2005. Retrieved 19 November 2005.
- ↑ "Importing or Bringing Medication into Japan for Personal Use". Japanese Ministry of Health, Labour and Welfare. 1 April 2004. Retrieved 3 November 2013.
- ↑ "Controlled Drugs and Substances Act". Canadian Justice Laws Website. Government of Canada. 11 November 2013. Retrieved 11 November 2013.
- ↑ "Table of controlled Narcotic Drugs under the Thai Narcotics Act" (PDF). Thailand Food and Drug Administration. 22 May 2013. Retrieved 11 November 2013.
- ↑ "Class A, B and C drugs". Home Office, Government of the United Kingdom. Archived from the original on 4 August 2007. Retrieved 23 July 2007.
- ↑ 186.0 186.1 186.2 "Identification". Lisdexamfetamine. Drugbank. University of Alberta. 8 February 2013. Retrieved 13 October 2013.
External links
| Wikimedia Commons has media related to amphetamine. |
- Template:PubChemLink (dextroamphetamine)
- Template:PubChemLink (racemic amphetamine)
- Template:PubChemLink (levoamphetamine)
- Comparative Toxicogenomics Database entry: Amphetamine
- Comparative Toxicogenomics Database entry: CARTPT
- U.S. National Library of Medicine: Drug Information Portal – Amphetamine
Template:Methamphetamine Template:ADHD pharmacotherapies Template:Catecholaminergics Template:Phenethylamines
- Pages with script errors
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Multiple names: editors list
- CS1 maint: Unrecognized language
- Pages with citations lacking titles
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Pages with broken file links
- Articles containing potentially dated statements from 2014
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Amphetamine
- Amphetamines
- Anorectics
- Attention deficit hyperactivity disorder
- Drugs acting on the cardiovascular system
- Drugs acting on the nervous system
- Drugs in sport
- Euphoriants
- Excitatory amino acid reuptake inhibitors
- German inventions
- Nootropics
- Norepinephrine-dopamine releasing agents
- Phenethylamines
- Stimulants
- TAAR1 agonists
- VMAT inhibitors